Alkynes – addition reaction of alkynes with hydrogen, halogen, hydrogen halides and water
Addition reactions: Like alkenes, alkynes undergo addition reactions due to their unsaturated nature. However, in alkynes, the addition occurs in two steps. In the first step, one molecule of the attacking reagent is added to the alkynes and its triple bond changes to a double bond. In the second step, a second molecule of the reagent is added which changes the double bond into a single bond and a saturated compound is obtained.
The alkene formed by the addition of to alkynes is generally trans-isomers. Some important electrophilic addition reactions are discussed below:
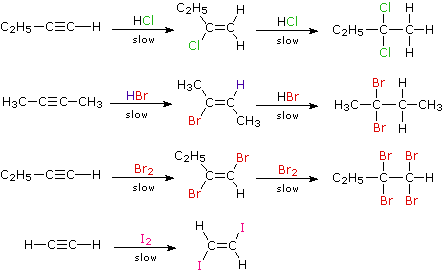
- Addition of halogens: halogens add to alkynes in two steps forming a dihalide and then tetrahalide. For example,
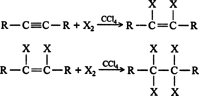
1,1,2,2-tetrachloroethane is called westron. It is used as a solvent.In the case of addition of bromine, the reddish-orange colour of the solution of bromine in carbon tetrachloride gets discharged as in alkenes. Therefore, this can be used as a test for unsaturation in alkynes also. Thus, the discharge of the brown colour of bromine indicates the presence of unsaturation.The reaction of alkynes with iodine is a very slow reaction. It is believed to stop after the addition of one molecule of iodine to alkene stage asThus, the order of reactivity of halogens towards alkynes is:
- Addition of halogen acids: the addition of halogen acids ( HCl, HBr, and HI) also takes place in two stages to form dihalogen derivatives. After one molecule of the acid has been added to a symmetrical alkyne, the product is an unsymmetrical derivative of alkane. The further addition takes place in accordance with the Markovnikov rule to form alkylidene dihalides in which two halogens are attached to the same carbon atom. For example,
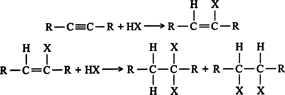
- Addition of hypohalous acid (HOX): alkynes react with two molecules of hypohalous acids (HOCl or HOBr) or halogen in the presence of water.
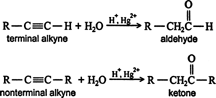
- Addition of water (hydration of alkynes): like alkanes and alkenes, alkynes are also immiscible and do not react with water. However, alkynes react with water in the presence of mercuric sulphate and sulphuric acid at 333 K. The products are carbonyl compounds (aldehydes and ketones). In these reactions, the first enol is formed which contains a double bond and an alcoholic group. Bit the enol formed is quite unstable in the acidic conditions. It isomerizes to a more stable (keto) form as a result of rearrangement. For example,
- Addition of hydrogen cyanide: alkynes react with hydrogen cyanide in the presence of barium cyanide catalyst. Acetylene gives vinyl cyanide or acrylonitrile which can be used to prepare synthetic fiber orlon.
- Addition of alcohols and carboxylic acids: ethyne adds a molecule of alcohol in the presence of alkali to give vinyl ether.
- Addition of hydrogen (hydrogenation): alkynes react readily with hydrogen in the presence of finely divided Ni, Pt or Pd as catalysts. The reaction is called as hydrogenation or reduction of alkynes.