In the world of chemistry, hydrocarbons are divided mainly into two categories. These include Aromatic hydrocarbon compounds and Aliphatic hydrocarbon compounds.
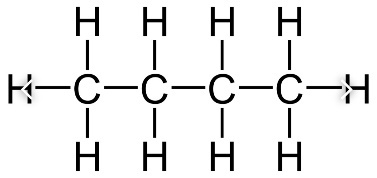
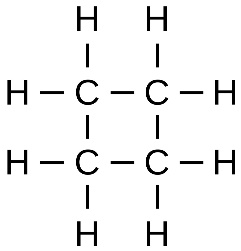
Aliphatic hydrocarbon compounds can be cyclic and non cyclic. Major organic compounds in organic chemistry are of cyclic nature. As this brings more stability to the compound. The most famous and especially stable compound of Aliphatic cyclic compound is the benzene ring.
Further, the Aliphatic Hydrocarbons can be divided into the aromatic compound and non aromatic compounds. The aromatic compounds are the cyclic compounds and the non aromatic compounds are the acyclic compounds.
The Aliphatic Hydrocarbon compounds could be saturated like hexane and unsaturated like hexene. In a saturated hydrocarbon compound, there are no double bonds. All the substituent atoms are bonded by single sigma binds. Whereas for the unsaturated compounds like hexene, there has to be a minimum of one double bond, that might be a covalent bond. Also, there may be a triple bond in the structure.
Properties of Aliphatic Hydrocarbon
The aliphatic hydrocarbons due to a difference in their molecular structure also differ in their properties. The varying empirical formula is due to the presence of single, double and triple bonds in the structures.
This could be seen in the differing characteristics of alkanes, alkenes and alkanes. The formation of double and triple binds is due to the catenation property of carbon. This results into the formation of complex compounds of aromatic hydrocarbons like benzene.
Alkanes with 10 or less than 10 carbon atoms are generally gases or liquids at the room temperature. Since the alkanes have a single bond, they are connected with weak Vanderwaal forces. This leads to low melting and boiling point of the alkanes. Whereas the boiling and melting point of the other compounds may increase due to an increase in the bond interacting strength.
The boiling point of hydrocarbons vary with molecular weight and branching in the compound. The higher the molecular weight, there is more increase in the boiling point of the Aliphatic Hydrocarbon. Whereas the lesser the branching in the compound higher is the boiling point.
Preparation of hydrocarbons
The general formula of Aliphatic hydrocarbon is CnH2n. Most of the preparation techniques include elimination reactions. The E2 elimination reaction and the E1 elimination reaction are the majorly used reactions.
The E2 elimination reaction is a single step process with 2nd order of kinetics. The degree of reaction in this process is 1°>2°>3°. This order is due to the steric hindrance in the compound. This reaction is most favoured in none polar aprotic solvents. The major yield from the reaction is the less substituted alkane.
The E1 elimination reaction is a two step process reaction. The order of kinetics of the reaction is 1st order. The degree of reaction of E1 elimination reaction is 1°<2°<3°. This is because of the carbonation stability of the compounds. The reaction is most favoured in the polar, protic type of solvent. Here in the rearrangement of products is possible. The most substituted product is the major product in the reaction.
Uses of aliphatic hydrocarbons
The aliphatic hydrocarbons are majorly used as fuels. The LPG and CNG are example of those. They serve as a purpose of lubricating agents and greasing purposes. The are also used in the manufacturing of polymers.
