The allotropes are different form of same element. It results due to the different bonding arrangements and the allotropes of the same elements have different chemical and physical properties. In the group of the carbon family, only the allotropes of carbon and Tin exist under normal conditions.
There are several allotropes of carbon or many different forms in which they exist. Interestingly the allotropes of carbon span a wide range of the physical properties. Diamond is the hardest substance that is occurring naturally, and graphite is one of the softest substances. Diamond is ultimate abrasive and transparent and it can be used as a thermal conductor and electrical insulator. However, graphite is a very good lubricant and is opaque. It is a thermal insulator and a good conductor of electricity. Some other allotropes of carbon include the buckyballs, glassy carbon, nanotubes, nanofoam, amorphous carbon and others.
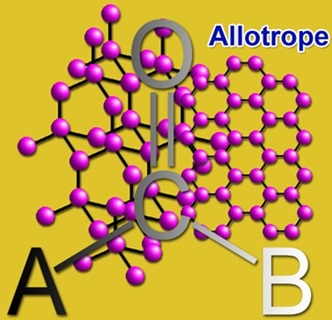
Carbon is capable to form the many allotropes and is structurally different from the others and this phenomenon is due to its valency. Earlier only graphite and diamond were known as the allotropes of carbon but now many more have been discovered. Some of the unusual forms of carbon are existing at the extreme pressures and high temperatures. At present, around 500 hypothetical 3-periodic allotropes are known for carbon. The hardness and depression of the light of the diamond make it a perfect choice for jewelry and industrial applications. Still, no naturally known substance can cut the diamond except another one.
There are two allotropes of silicon, existing at the room temperature. These allotropes are known as crystalline and amorphous allotropes. The amorphous allotrope is gray in color and it has a metallic luster. The amorphous allotrope is like brown powder. It is possible to grow the single crystals of silicon by a process called as the Czochralski process. These crystals can be doped with other elements such as the germanium, phosphorus, boron, gallium, and arsenic and are used for the manufacturing of the solid-state electronic devices such as solar cells, rectifiers, microchips, and the transistors.
Two allotropes of tin are known and they are α-tin and β-tin. Typically, tin is found in the β-tin form that has the looks like silvery metal. At standard pressure, β-tin is converted to the α-tin that is the gray powder at the temperature below the 13.25 degree Celsius. It can cause the Tin, to object in the cold temperatures, to crumble into the gray powder in a process of tin rot or tin pest. The structure of β-tin is distorted and is like the closed pack lattice while α-tin, has a diamond-like structure.
Under standard conditions, germanium is the silvery metal, semi-metallic and brittle element. It constitutes an allotrope known as α germanium that has a metallic luster and a structure similar to that of the diamond cubic crystal structure. When the pressure exceeds the 120 Kbar then it becomes the β germanium allotrope and its structure is the same as the β germanium.
