Nitration is a type of substitution reaction of aromatic hydrocarbon. In these reactions, one or more hydrogen atoms in the benzene ring get replaced by other atom or groups. In the context of organic compounds, let us first understand the importance of nitrogen. Nitrogen is necessary for many days to day things we depend on like pharmaceuticals, textiles, plastics and the biological polymers such as proteins. Hence organic compounds which contain nitrogen atoms are so important we should understand the chemical reactions that introduce nitrogen atom to other substrates. One of the most important ways to add nitrogen to other organic substrate is an electrophilic substitution reaction called nitration.
Nitration: the replacement of hydrogen atom in the ring of an arene by a nitro group is called nitration. It is carried out by heating benzene with the nitrating mixture consisting of concentrated nitric acid and sulphuruic acid to about 330 K.
In order to nitration reaction to take place, we need two things an electrophile and a nucleophile. In the electrophilic aromatic substitution reactions the nucleophile is always the aromatic ring itself, and in this case, the electrophile actsually the nitro group. As the word aromatic itself implies that an aromatic ring is needed for this reaction, and the π-electrons inside the ring will behave as a nucleophile. Now about substitution, in this nitration, we will be substituting a hydrogen atom of the aromatic ring with the nitro group. we also need an electrophile to nitration to successfully happen. Let us talk about the mechanism of this reaction.
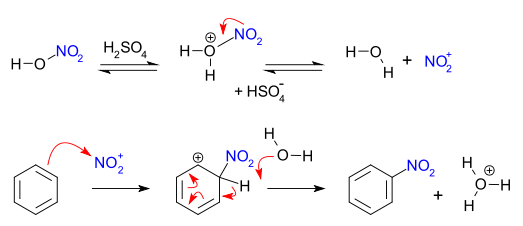
Mechanism of nitration of benzene: in the nitration of benzene, a hydrogen atom of benzene is substituted by a nitro group. The attacking reagent is nitronium ion, whose formation is facilitated by the presence of sulphuric acid(catalyst). The mechanism is illustrated in the following steps:
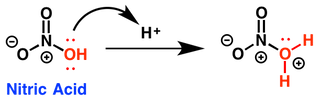
- The electrophile nitronium ion is generated as follows: A nitrogen electrophile is generated by taking nitric acid and suphuric acid and adding them together. When we mix these two reagents a series of events takes place that provides with the electrophile we need for nitration. First of all, the hydroxyl group (OH) on a molecule of nitric acid is protonated by a hydrogen ion which is provides by the suphuric acid. As a result, the water molecule is generated which is a very good leaving group. The water molecule falls off, which provides an electrophile called nitronium ion, which reacts with the aromatic ring.
- The electrophile attacks the benzene ring to form an intermediate carbocation. Let us see how this happens, the set of pi-electrons from our aromatic ring comes out and makes a bond with the nitronium ion. We need to break an existing nitrogen-oxygen bond because nitrogen can’t have more than four bonds. It is also noted that when the carbon-carbon double bond breaks the one of the carbon is short 2 electrons, which is why it has a positive charge.
- The intermediate carbocation ion loses
ion and results in the formation of a nitro compound. The intermediate carbocation which formed is very reactive because it no longer remains aromatic. It doesn’t stick around long, and in the case, a water molecule is sufficient enough of a base to come in and pluck off hydrogen atom, which regenerates aromaticity in the ring system and gives the nitrated product.