Taking the help of valence electrons, metals conducts electricity. Metals’ atomic orbitals or electron cloud have the same energy which combines forming molecular orbital whose energy is closer to one another in order to form the ‘band’. In condition when the band is filled partially or it overlaps with another high energy-free (unoccupied C.B i.e. conduction band, the electrons with an easy effort can flow under the electric field which exerted or applied, and which shows high conductivity. So, in this we are taking a example of sodium (Na).
The atomic or electronic configuration of Sodium is – “1s2, 2s2, 2p6, 3s1.” there’s one unpaired electron in 3s orbital of Na. So, 3s valence electrons in the atomic orbitals of Na overlaps alongside this kind of orbital, having the same energy for the formation of molecular orbits.
Carrying on in the process, atomic orbitals combines in the similar fashion and thus forms a band. This band’s “energy spread” is determined by the energy difference present between “antibonding orbital’s” highest energy and strongest restrained “bonding orbital.”
In different cases, when the energy band gap between the conduction and valence band is high enough, the electrons however just fails to jump to conduction band from the valence band. Alike these compounds, they exhibit much less or negligible conductivity. For eg.: in glass – when the energy band gap between the conduction and valence band is tiny in size, a few of the electrons might have a jump to conduction band from the valence band and hence displays some conductivity. These types of substances are called the semiconductors. Eg: Si, Ge (Silicon and Germanium respectively).
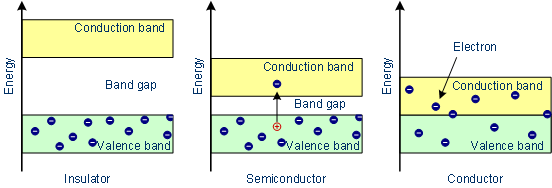
Fig: (Conductor in the above image can be taken as metal)
Metal Case: It’s quite clear from the above image – there’s no separation in between the bands, in metal or conductor case. This results in helping the triggered electrons to quite easily to change its location from one orbital to another orbital and thus, metals are known to be good conductors of electricity.
Semiconductor Case: There’s a short band gap present in between conduction band the valence band. Therefore, when the trigger or incitation process is done, then electron’s small fraction with sufficient energy jumps.
Nevertheless, the conductivity of these substances can be increased by accelerating the doping or the temperature.
Insulators Case: The band gap energy or the forbidden energy gap is very high in between conduction band and valence band. This is the reason why there’s no conductivity present in such substances.
