Binding Energy per Nucleon and its Variation with Mass Number
The binding energy per nucleon of the nucleus is the specific binding energy divided by the number of nucleons in total. The nuclei are made up of protons and neutrons but the actual mass of the nucleus is always less as compare to the mass its components that are constituting it. This difference is accounting for the measurement of the nuclear binding energy that enables the holding of the nucleus together. Binding energy per nucleon can be calculated by using the Einstein equation.
E= mc2
Nuclear binding energy = Δmc2
It is possible to explain the enormity of nuclear binding energy by comparing it to the binding energy of the electron of the atom. Binding energy per nucleon is the most significant experimental quantity in nuclear physics. This can be defined as follows
BEN= Eb/A
This quantity is the amount of average energy that is required for removing the individual nucleon from the nucleus of the atom. It is analogous to the ionization energy of the electron of an atom. Relatively, if the amount of BEN is large, then it is the indicator that the nucleus is relatively stable. The values of BEN can be estimated by nuclear scattering experiments.
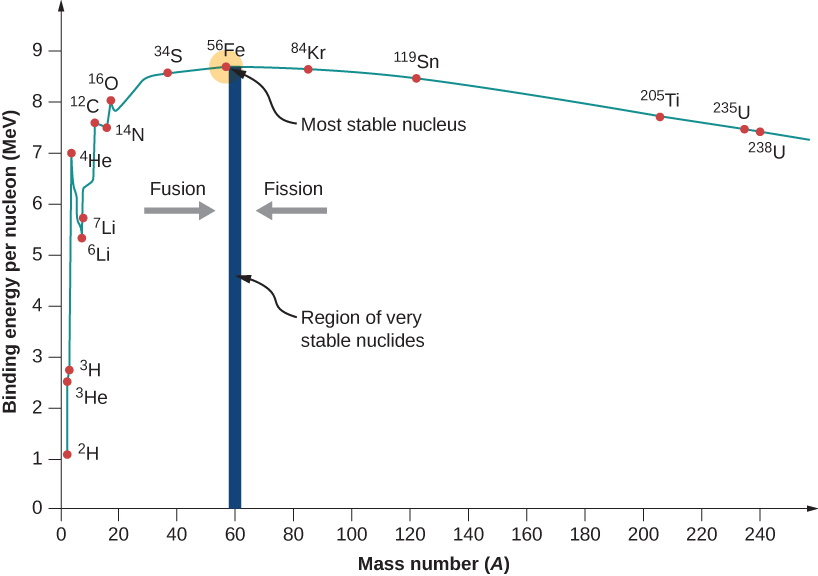
In the graph shown above, the atomic number versus the binding energy per nucleon is given. Many physicists are considering this graph, and in physics, this is one of the most important graphs. There are two notes in order, first is that typically the BEN values are ranging from 6 to 10 MeV. In other words, it can be said that the several million electron volts are required to attack the nucleon from its typical nucleus. It can be compared to that 13.6 eV is required for ionizing the electron in the ground state of hydrogen. Due to this reason, the nuclear force is also referred to as a strong nuclear force.
Variation with Mass Number
Secondly, the graph is rising at low point A, and its peak is very near to the iron. Later on, it is tapering at the high A. The peak values are suggesting that in nature, the nucleus of iron is most stable. Due to this reason, the graph is rising high and tapering off as there are competing forces in the nucleus. By moving from light nuclei to heavy nuclei, the overall binding energy and release of energy are increased.
