Bohr model or Rutherford-Bohr model is a system consisting of a dense nucleus which is surrounded by orbiting electrons it is similar to the structure of the solar system. Though its structure is same like the solar system but it is attracted by electromagnetic force in place of gravity. This model is based on the hydrogen atom and also compared with the valence shell atom. As a theory, it is known as the first-order approximation of the hydrogen atom which uses broader and accurate quantum physics. This model is also recognized as the simplest model and it is used to introduce quantum physics to the students who are new in this world. It has its own points which are proven correct by both of the scientists and those points are so much relatable.
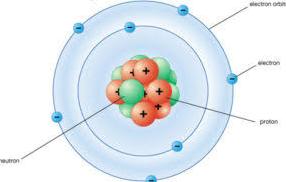
What are the applications put by Bohr?
Before these particular models, many models with more diversification were put forth but not a single one is able to fulfill what it requires. He put forward three main and important populated that easily sums up all applications of the model. The first one is that the electron is able to move in a particular angle around the nucleus also without radiating any energy which is contrary to what classic electromagnetism suggests. Second is the stationary orbits are a particular distance which makes angular momentum of the revolving electron an integral multiple of reduced Planck constant and in this Bohr is able to count the energies allowed orbits of the hydrogen atom and another atom like hydrogen. This model of Bohr is based on Planck’s quantum theory of radiation. The third and most important application he suggested was the electrons can only gain and lose energy only by jumping from one orbit allowed to the other absorbing and emitting electromagnetic radiation.
What are the shortcomings of this model?
Every mode every experience go through a time where there is no process or nothing is finalized and some of them may have a shortcoming in the process. This model of Bohr gives incorrect values for the ground state orbital angular momentum as per the suggestion or the experiment ground states is known to be zero for the experiment. There are so many theories that failed to explain the system of energies this model also lacks in stating the lowest energy state is spherically symmetric and does not point any explanation in that part of the model.
