Alkynes – chemical reactions
The alkynes have at least one triple bond in them, therefore, they are quite reactive chemically. They readily take part in addition reaction and can also be easily oxidized, a few important chemical reactions of alkynes are discussed below:
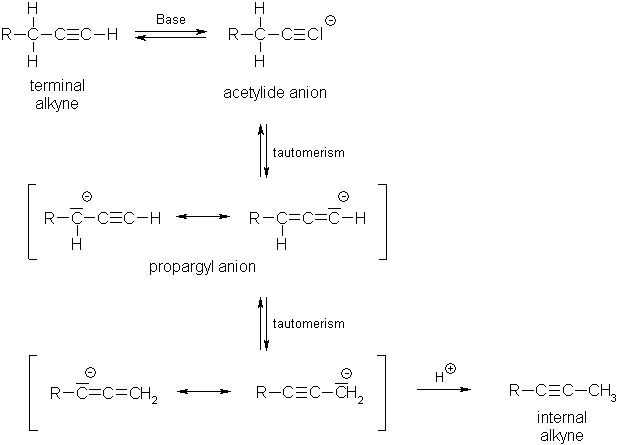
- Acidic character of alkynes: Acetylene and other terminal alkynes are weakly acidic in character. They react with strong bases like sodium metal at 475 K or sodamide, to form sodium acetylide derivatives known as acetylides or alkynides with the liberation of hydrogen gas as shown here:
These reactions have not been observed in the case of alkenes and alkanes indicating that alkynes are acidic in nature in comparison to alkenes and alkanes.
- Addition reactions: Like alkenes, alkynes undergo addition reactions due to their unsaturated nature. However, in alkynes, the addition occurs in two steps. In the first step, one molecule of the attacking reagent is added to the alkynes and its triple bond changes to a double bond. In the second step, a second molecule of the reagent is added which changes the double bond into a single bond and a saturated compound is obtained.
The alkene formed by the addition of to alkynes is generally trans-isomers. - Oxidation: Alkynes can be oxidized under different conditions to give a variety of products.
- Combustion: When alkynes are burnt in air or oxygen, carbon dioxide, and water are formed. The reaction is highly exothermic and a large amount of heat is produced.
Under normal conditions, acetylene burns with a luminous yellow sooty flame due to the presence of higher carbon content. However, with air or oxygen under high pressure, acetylene burns with a blue flame producing a high temperature of the order of 300 K. The heat produced during the combustion of acetylene can be used for welding purposes in the form of oxy-acetylene flame.
- Oxidation with alkaline potassium permanganate:
- With a dilute cold
solution: alkynes react with dilute cold alkaline
solution and form diketones or carboxylic acids depending upon the nature of alkynes. In these reactions, ≡CH part of the alkyne is oxidized to –COOH group while the RC≡ part is oxidized RCO- part. For example,
In the above reaction, the pink colour of an alkaline(bayer’s reagent) gets discharged. Therefore, this reaction can be used as a test for alkynes.
- With hot
solution:
When alkynes are treated with hotsolution, the triple bond is completely broken leading to the formation of carboxylic acids and carbon dioxide depending upon the position of the triple bond. The cleavage occurs at the site of the triple bond.In this case, ≡CH part of the alkyne is oxidized to
while the RC≡ part is oxidized to RCOOH. For example,
- Oxidation with ozone (ozonolysis): the alkynes react with ozone to form ozonides. These ozonides on decomposition with zinc dust and water or
give dicarbonyl compounds.
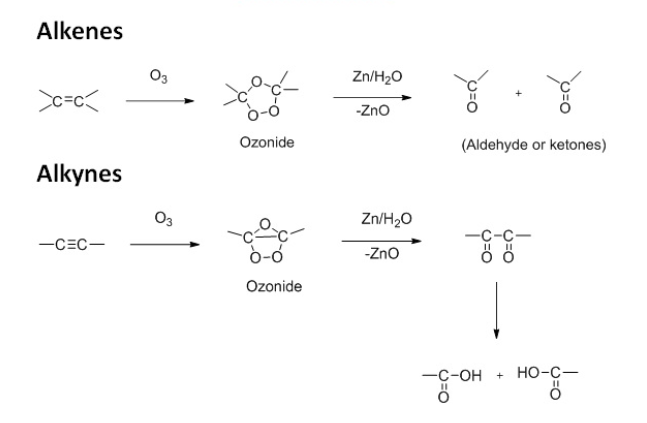
- With a dilute cold
- Combustion: When alkynes are burnt in air or oxygen, carbon dioxide, and water are formed. The reaction is highly exothermic and a large amount of heat is produced.
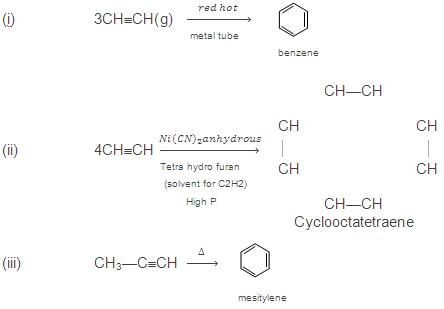
- Polymerization reactions: alkynes have a larger tendency to polymerize than alkenes and therefore undergo two types of polymerization reactions:
- Linear polymerization: acetylene undergoes linear polymerization under suitable conditions to form linear polyacetylene or poly ethyne.
- Cyclic polymerization: alkynes on passing through a red-hot iron tube at 873 K undergo cyclic polymerization.
- Isomerization of alkynes: when alkynes are heated with sodamide in an inert solvent, such as kerosene oil or paraffin oil, they undergo isomerization.