Chemical energy which is in stored condition by molecules can be spreaded as heat during chemical reactions. The branch of chemistry which studies the quantitative relationship between heat and other forms of energy is defined as thermodynamics. The laws of thermodynamics examine the energy changes of macroscopic systems with a large number of molecules and do not emphasize on microscopic properties. Hence, thermodynamics is only concerned with large scale observations. Thermodynamics are not amphasized on how or what rate these energy transformations are carried out but its based on initial and decidable states of a system undergoing the differences. These laws are applied when a system is in equilibrium or changes its place from one state to another.
Likewise, the laws of thermodynamics deal with energy changes during a reaction and are not concerned with the rate at which the reaction is proceeding.
Thermodynamics has become part of our life. Whether travelling in a car, sitting comfortably in an AC room or enjoying a cold drink from the cooler, the applications of thermodynamics almost everywhere. Heat pumps, air conditioners, and refrigerators use to transfer heat from cold to warmth. We often use some general terms like work, heat and energy to express thermodynamics of any system. Classical thermodynamics provides a direct and easy solution for engineering problems
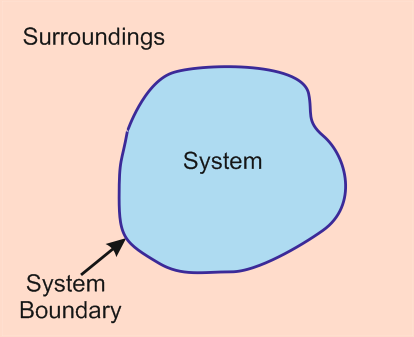
Figure 1: A thermodynamic system is surrounded by a boundary and surrounding within a universe.
The universe = The system + The surroundings
A system refers to a part of the universe that is under observation and the surroundings are everything else in the universe except the system. A boundary is a wall that separates the system from the surroundings. The collective behaviour of a large number of atoms, molecules or ions is associated with macroscopic properties, namely extensive and intensive properties. rigorous properties are self supporting of the mass of the system. These properties include temperature pressure and density. The value of extensive properties depends on the size and extent of the system while the specific properties are extensive properties per unit mass of a system.
A system is under Thermodynamic equilibrium when none of the state variables is changing and it satisfied three types of equilibriums.
- Mechanical equilibrium is achieved with no mechanical motion and no change in pressure or volume of the system.
- Thermal equilibrium is achieved when there is no flow of heat and the temperature of the system remains constant with change in time.
- Chemical equilibrium is achieved when the rate of the forward reaction is equal to the backward reaction and the overall moles of the system is constant
State variables are used to describe the condition of a system. These variables include pressure, temperature, volume, internal energy, number of moles etc. The state of the system changes when any one or more of these variables are altered. Macroscopic properties like pressure and temperature do not change with time for a system in an equilibrium state.
