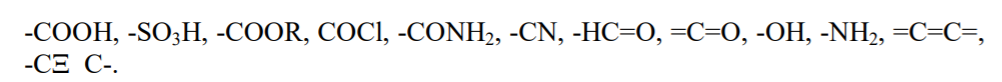A large number of existing organic compounds and their ever-increasing number with time have made it necessary that we classify them. This is done on the basis of their structure. Organic compounds are classified as follows:
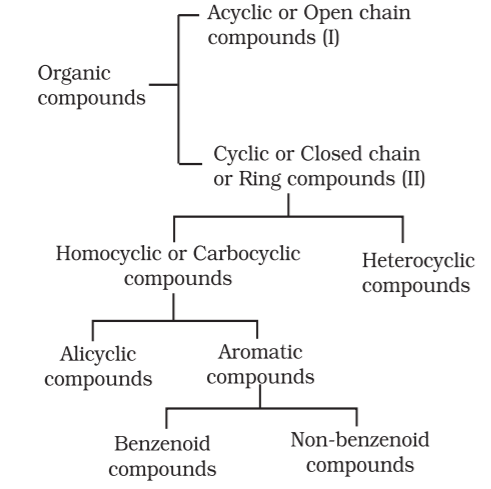
Open chain compounds or Acyclic:
These organic compounds are also called as aliphatic compounds. This consist of straight or branched chain compounds, for example:
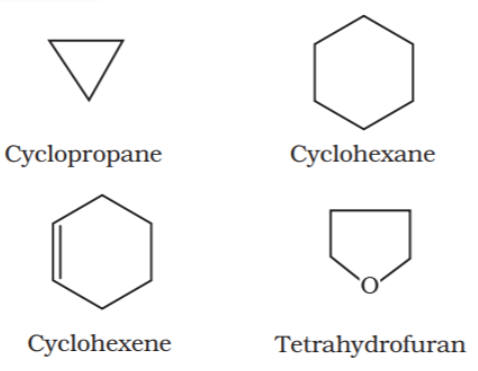
Alicyclic or closed chain or ring compounds:
Closed chain compounds are compounds in which carbon atoms joined in the form of a ring. Sometimes atoms other than carbon are also present in the ring (heterocyclic). Some of the examples of this type of compounds are as follows:
Some of the properties of these compounds are similar to those of aliphatic compounds.
Aromatic compounds:
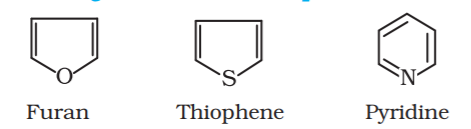
These are special types of compounds and include benzene and other related ring compounds (benzenoid). Aromatic compounds may also have hetero atom in the ring like alicyclic compounds. Such compounds are called heterocyclic aromatic compounds. Following are the examples of various types of aromatic compounds:
Benzenoid aromatic compounds:
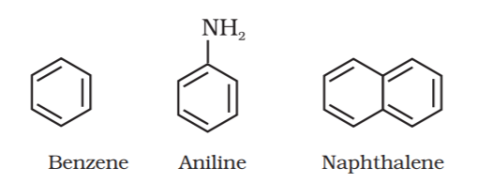
Non-benzenoid compound:
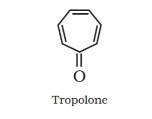
Heterocyclic aromatic compounds:
Nomenclature of Organic compounds.
Millions of compounds are dealt by Organic chemistry deals. Therefore there identification becomes very important. To identify them clearly, a systematic method of naming has been developed. It is called as the IUPAC (International Union of Pure and Applied Chemistry) system of nomenclature. In this systematic nomenclature, the names are dependent on the structure of the compound. Due to this the reader or listener can deduce the structure from its IUPAC name.Before the IUPAC system of nomenclature came into existence, organic compounds were assigned names based on their origin or certain properties.
A systematic name of an organic compound is generally derived by the identification of the parent hydrocarbon and the functional groups attached to it. For example:
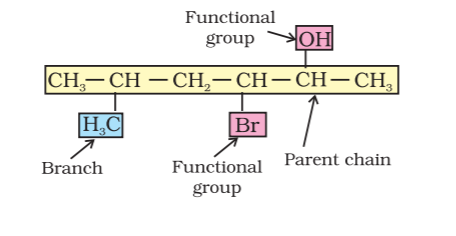
The parent name can be modified to obtain the actual name, by further using prefixes and suffixes
IUPAC Nomenclature of Alkanes:
Straight chain alkanes:
The names of these compounds is based on their chain structure, and end with ‘-ane’ suffix and carry a prefix which indicate the number of carbon atoms present in the chain.
Branched chain hydrocarbon:
- Very first thing to do is to identify the longest carbon chain in the molecule.
- The numbering is done in a way such that the branched carbon atoms get the lowest possible value.
- The names of the alkyl groups attached to a branch are then prefixed to the name of the parent alkane. Its position is indicated by numbers.
- The lower number is given to the one which is first in alphabetical order.
- The carbon atom of the branch that is attached to the root alkane is numbered 1.
Organic compounds having functional groups:
The longest chain of carbon atoms containing the functional groups is numbered in a way that the carbon atom attached to the functional group gets lowest number in the chain. Where there are more functional groups then a priority order is followed as: