Surface Chemistry: Coagulation
Physical and chemical processes to induce collisions and growth of particles, and to remove some soluble contaminants:
Coagulation: Formally, means making particles “sticky” (rapid mix step); colloquially, also refers to forming new particles that collect dissolved contaminants
Flocculation: Collisions among sticky particles (slow mix step), to converge and form fewer, larger and visible particles.
Coagulation is a process that brings colloidal particles together to change into large-sized particles that ultimately settle as a precipitate or float on the surface .It is generally conducted by addition of electrolytes.
Colloidal particles stay suspended for a long time since they are charged by adsorbing ions from the dispersing medium onto their surface while the colloidal system remains neutral. Due to the repulsion of the like-charged particles, the particles do not from forming heavier aggregates with a greater settling tendency .The coagulation power or flocculation value is a parameter that signifies the minimum amount of an electrolyte that must be added to one litre of a colloidal solution so as to bring about complete coagulation or flocculation respectively. Likewise, smaller flocculation value of an electrolyte indicates towards a greater coagulating or precipitating power.
A colloidal system can be destroyed or coagulated by the addition of electrolytes. When an electrolyte is added to a colloidal solution, the particles of the sol take up the ions which are oppositely charged and thus get neutralized. These neutral particles accumulate to form particles of a larger size globule. The added ions from the electrolyte then neutralize the charged colloidal particles. Accordingly, particles clump together forming heavier aggregates that settle out from the dispersion.
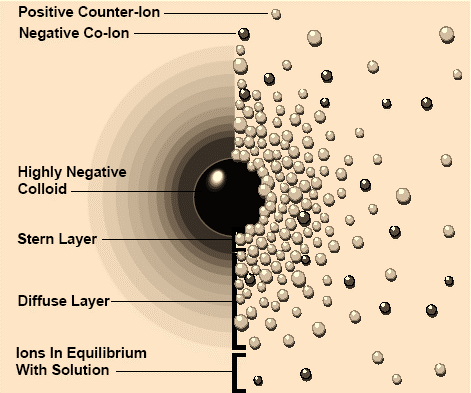
Fig 1: Particles attract opposite-charged ions, and repel like-charged ions, generating a diffuse layer in which the particle’s charge is neutralized
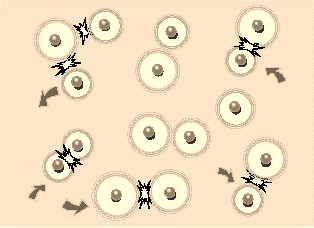
Fig 2:When the surface potentials are high, the repulsion is strong even at large separations, and particles do not collide
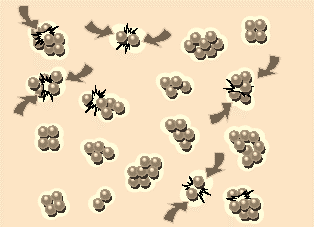
Fig 3: When the surface potentials are low, the particles can approach close enough to collide and stick
Hardy Schulze law indicates the quantity of the electrolyte required to coagulate a definite amount of a colloidal solution. This quantity depends on the valency of the ion with a charge opposite to that of the colloidal particles.
It can be defined as
The rate of coagulation increases with higher valency of the oppositely charged ion of the electrolyte being added in the mixture. Likewise, the coagulating power of an electrolyte accelerates rapidly with increasing value of the valency of cation or an anion in the electrolyte.
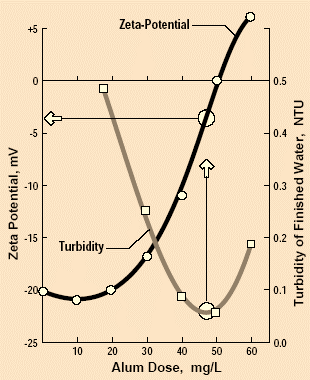
Fig 4: The “zeta potential” is an indicator of the surface electrical potential. When it is zero, the particles have the greatest tendency to collide, and they are therefore easiest to remove.
In conclusion, the coagulation power of negatively charged arsenious sulphide sol., trivalent cations is far more than divalent cations, while the divalent cations are more effective than monovalent cations. Similarly, for coagulation of positively charged ferric hydroxide sol, tetravalent anions are more effective than trivalent anions which are more effective than divalent anions which in turn are more effective than monovalent anions.
For negatively charged sol, the coagulating power trend is as follows:

For positively charged, then the coagulating power trend is as follows:
