The breaking of bonds between the atoms, either with plentiful oxygen or limited oxygen is what Combustion of hydrocarbon is all about. The products formed out of this reaction are water and CO2.
Depending on the amount of oxygen, combustion is explained as follows:
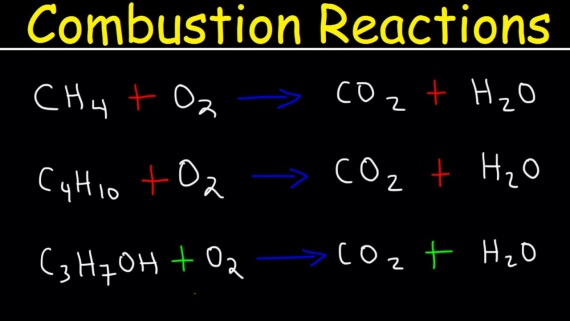
Complete combustion
Complete combustion takes place in the presence of plentiful oxygen. Complete combustion of any hydrocarbon leads to the production of carbon dioxide and water. It is quite important that you write very balanced equations for these reactions. As they often come up as a part of thermochemistry calculations. Some are easier than others. For example alkanes containing even number of carbon atoms are marginally harder than those with an odd number.
Ex. Propane Combustion
With propane (C3H8), you can balance the carbons and hydrogen as you write the equation down. Your first draft would be:
C3H8+O2→3CO2+4H2O (1) (1) C3H8+O2→3CO2+4H2O
Counting the oxygen atoms leads directly to the final version:
C3H8+5O2→3CO2+4H2O
Ex. Butane Combustion
With butane (C4H10), you can again balance the carbons and hydrogen as you write the equation down.
C4H10+O2→4CO2+5H2O (3) (3) C4H10+O2→4CO2+5H2O
Counting the oxygen leads to a slight problem – with 13 on the right-hand side. The simple trick is to allow you to have “six-and-a-half” O2 molecules on the left.
C4H10+61/2O2→4CO2+5H2O (4) (4) C4H10+61/2O2→4CO2+5H2O
If that offends you, double everything:
2C4H10+13O2→8CO2+10H2O
It becomes harder to ignite the hydrocarbons as the molecules get bigger. This is because the problem in vaporizing faced by bigger molecules. If the oxygen and the hydrocarbon are well mixed as gases, the reaction is much better. Only those molecules on the surface can react with the oxygen if the liquid is not very volatile. Bigger molecules have greater Van der Waals attractions which make it more difficult for them to break away from their neighbours and turn to a gas.
All the hydrocarbons will burn with a blue flame provided the combustion is complete. However, as the number of carbon atoms in the molecules rises combustion tends to be less complete. That means, you are more likely to get a yellow, smoky flame bigger the hydrocarbon.
Incomplete combustion
Incomplete combustion takes place where there is not enough oxygen present. It can lead to the formation of carbon or carbon monoxide. When it comes to hydrocarbons, the hydrogen gets the first chance at the oxygen, and the carbon gets whatever is left. The glowing carbon particle’s presence in a flame turns it yellow. The black carbon is often visible in the smoke. Carbon monoxide is produced as a colourless poisonous gas.
Why carbon monoxide is poisonous: Oxygen is carried around the blood by haemoglobin, which unfortunately binds to exactly the same site on the haemoglobin that oxygen does. The difference is that carbon monoxide binds irreversibly (or very strongly) – making that particular molecule of haemoglobin useless for carrying oxygen. If you breathe in enough carbon monoxide you will die from a sort of internal suffocation.
Trends in combustion:
For a hydrocarbon
If complete ignition occurs —-> it burns with a blue flame.
With the increase in the molecular mass of hydrocarbon——-> it starts burning with a yellow-colored flame. This shows incomplete burning.
With increasing molecular weights, the burning of hydrocarbons becomes difficult.
