For each matter, mass is the basic physical property. Atomic mass is the sum of all masses of electrons, neutrons, and protons, in an atom. As compared to the mass of neutrons, and protons, the mass of electrons is much less. For the elements, the atomic mass is the average mass of atoms of the element and it is measured in the atomic mass unit (amu) also called Dalton. It has significant use, for finding the average masses of molecules and atoms for solving the stoichiometry problems.
Historical Reasons for Naming Atomic Mass
The name atomic mass is given due to the historical reasons, and it was originated from the fact of investigation, of the physical objects on the microscopic and macroscopic levels. Additionally, this situation is more complicated due to the isotopic distribution. On the macroscopic level, most of the mass measurements of the pure substances is referring to the mixture of isotopes. It indicates that the mixture is not pure according to the physical point of view. For example, there is no correspondence between the microscopic and macroscopic mass of oxygen.
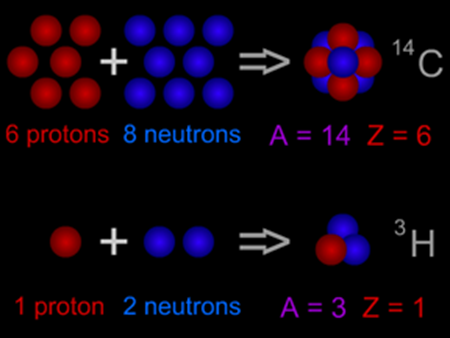
The method for finding the atomic mass, is dependent on the purpose, that either one has to look at the natural sample, single atom, or the atom containing some ratio of isotopes. Atomic mass represents the quantity of matter that is contained in the atoms of an element. As compared to the sum of the masses, the atomic mass is slightly less. This difference is obvious and is known as the mass defect and it is accounted for due to the reason of a combination of particles by the conversion into the binding energy.
Mass and Weight Should not be Confused with Each Other
In general, this mass is the weighted average, of all the isotopes of the elements, where the mass of each isotope is multiplied by the abundance of this specific isotope. Sometimes, it is also referred to as atomic weight, but using the term mass is the most accurate. Most probably, for the measurement of various objects, they are weighed in the grams and kilograms, but the atom is the extremely small quantity and its mass cannot be measured in these units. The concept of mass is recently distinguished from the weight. But in the colloquial language, it is still doubtful. Many people are using term weight instead of mass, but these two terms are different. For the objects, mass is the fundamental property but weight is a force. Weight is the acting force that is exerted on the mass, due to the gravitational pull.
