We all are familiar with a famous scientist, James Chadwick. He was the scientist who first introduced neutrons, and stated that the mass of neutrons is the same as the protons with little difference in them. Also, he mentioned that the pair of neutron and proton in the nucleus of an atom is called the nucleon. Due to these discoveries, the atomic number, mass number, and several neutrons are well understood by scientists. So, the main discovery of not only the Isotones but of Isobars and Isotopes is due to this scientist. This is how the discovery took place.
What are Isotones?
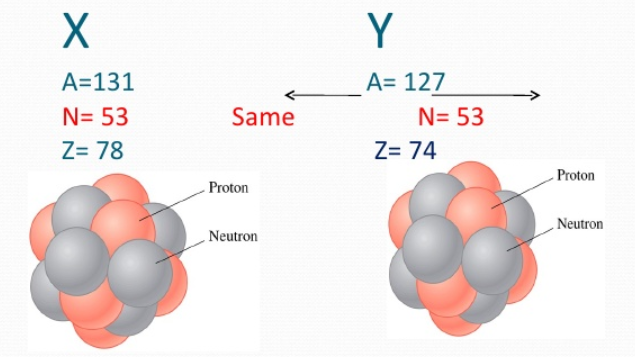
Isotones are simply the elements in the periodic table that have nuclides with a different number of neutrons, but the number of protons in them is essentially the same in number. For instance, we can take the example of Boron and Chlorine. In the case of Boron-12 and Chlorine-13, you might see the different number of neutrons, but the number of protons in them is the same, which is 7. There are so many other examples for it which include potassium, calcium, Sulphur, argon, chlorine, etc.
The term was first introduced by a German Physician, but the origin of the word is Greek that means Stretching. For the stability of isotones, you can see that it is much more than that of the isobars which are less stable compared to isotones. These are the basics of isotones which can make the basic idea in mind about what they are.
Are Isotones different from Isobars?
As you know that the isobars have the same mass number, but the atomic number is different between two nuclei of the elements. However, the isotones are those that have the same number of neutrons. So, you can see and know that these two are completely different from each other.
It is a very bog confusion that most of the people think that they are the same and then try to use these terms alternately, which is completely wrong. The difference is quite clear from the definition of both isobars and isotones, and that is enough to maintain a difference between them in your head. All you need to know about Isotones is that this term is introduced at the end. After the discovery of Isotopes, then the Isobars, the scientists came to know about the Isotones. So, there is a difference between these terms. Although the difference is little, it is there for sure.
