All the atoms of elements are not identical, and some atoms of the same elements are having a different number of neutrons. The different versions of atoms of the same elements are known as isotopes. Atoms are composed of neutrons, protons, and electrons. The neutrons and protons are present in the nucleus, and electrons are revolving around the nucleus.
Description
For understanding the concept of isotopes, let consider a pair of identical twins, but these twins are having a different temperament. Due to their twin nature, close and careful analysis is required for understanding. In the term of physics, these twins can be considered as isotopes of each other. For the given elements, the number of neutrons can vary from each other, while the number of protons remains the same. Such kinds of different versions of the same elements are known as the isotopes. As atomic mass is equal to the number of neutrons and protons, and atomic number is equivalent to the number of protons, so it can be said that the isotopes are having different mass numbers but the same atomic numbers.
Examples of Isotopes
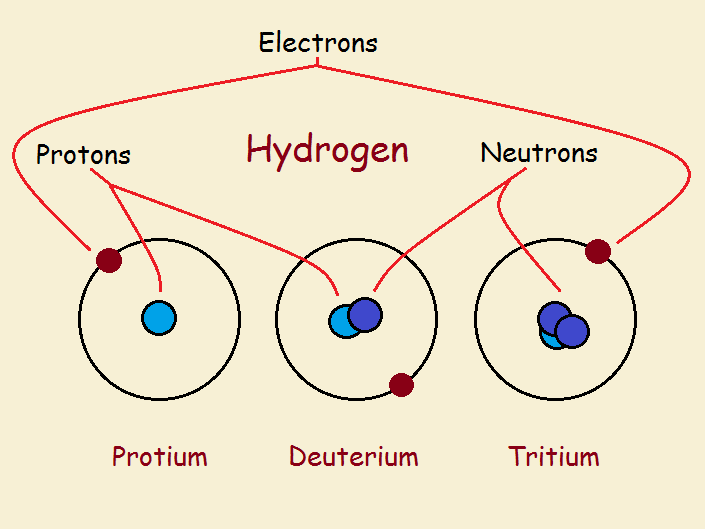
Hydrogen is having three stable isotopes, all of them are having the same number of protons, but varying numbers of neutrons. There are no neutrons in the protium. In deuterium, there is only one neutron and two neutrons are present in the tritium. Similarly, carbon is also having three isotopic forms, such as carbon 12, carbon, 13, and carbon 14. These numbers are representative of atomic masses of isotopic forms of carbon. The atomic number is a unique property that is important for the determination of elements. So, the atomic number is constant for all isotopic forms. For the element of carbon, only carbon 12 is stable, whereas, carbon 14 is the radioactive isotope. 81 stable elements are having approximately 275 different isotopes. Currently, more than 800 synthetic and natural isotopes are known.
Characteristics of isotopes
For each particular element, the physical properties of isotopes are varying from each other. It is evident from the fact that physical properties are dependent on their mass. Mass of the isotopes is different from each other. The isotopes can be separated from each other by using the process of diffusion and fractional distillation. Carbon isotopes have significant uses in carbon dating. Radioactive isotopes have various medicinal applications. Isotopes of uranium have significant uses in the nuclear reactors, and the isotopes of cobalt and iodine are used for the treatment of cancer, and goiter respectively.
