The composition of the nucleus can be described by the two main hypotheses, such as the proton-neutron hypothesis, and the proton-electron hypothesis. The nucleus of the atom is containing protons, and neutrons, while the electrons are revolving around the nucleus. In the atomic nucleus, the positively charged protons are close to each other. At the same time, the repulsive forces are also tremendous. The binding of protons and neutrons is due to strong forces of attraction. Without the presence of stronger forces, the positively charged protons will be blown away.
Size of the Nucleus
The diameter of the nucleus is 1.6 fm (10−15 m) for the lighter most and 15 fm for the heavier most atoms. As compared to the size of an atom, the size of the nucleus is much smaller. Despite its smaller size, it is having the larger most mass of the atom.
Isotopes and Nucleoids
The atomic isotopes are based on the number of neutrons in the nucleus of the atom. The isotopes of the same elements are having almost similar properties. The type of nucleus can be determined by the number of neutrons and protons. The mass of protons and neutrons is nearly the same, and by combining their number, the atomic mass of the atom can find out easily.
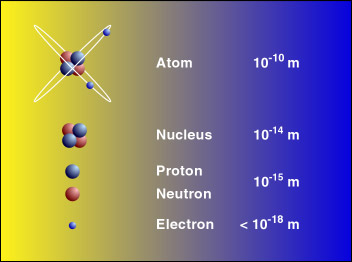
Distribution of Particles in the Nucleus
The protons and neutrons are tightly packed in the nucleus. In an atom, these particles are most heavy and due to this reason, the 99.9%, the mass of the atom is concentrated in the nucleus. The nucleus of the atom is having a positive charge due to the presence of protons. The nucleus is stabilized due to the presence of electrons that are revolving around it. Due to mass concentration at the nucleus, it is immense that there is the existence of larger nuclear forces that are holding protons and electrons. The protons of the atoms are in closer vicinity to each other, and they are present inside the tiny nucleus, so the presence of electrostatic forces is also evident.
Until, 1932, it was considered that the nucleus is having only protons. In terms of mass concentration, most of the space in the atom is empty. After twelve years, a postulate was given by Lord Ernest Rutherford, about the existence of the neutral particle in the atomic nucleus. The results of the research conducted by James Chadwick proved the existence of the neutron. By this discovery, the atomic nucleus model was changed.
