p-Block Elements: Group 17
Compounds of halogen
Hydrogen halides are made up of binary compounds of halogens and hydrogen. They form strong hydrohalic acids in water, except HF. The hydrogen-fluorine bond is highly polar because fluorine is much more electronegative than hydrogen, and each fluorine atom in HF has three lone pairs of electrons. The hydrogen atom, therefore, has a partial positive charge and the fluorine atoms have a partial negative charge. A lone pair of electrons on the fluorine atom in one molecule is attracted to the δ+ hydrogen atom in a neighboring molecule. This increases the strength of intermolecular forces, which increases the boiling point. The bonds in the other hydrogen halides are less polar because the other halogen atoms are less electronegative than fluorine. The lone pairs on the other halogen atoms are also in higher energy levels so their charge is less concentrated. Hydrogen halides can be made directly from the elements. Mixtures of H2 and Cl2, react with explosive violence in the presence of light as a catalyst to form HCl.
Chemists are usually more interested in aqueous solutions or mineral acids of these compounds than the pure gases. These compounds are usually synthesized in water and minerals. For example, HCl is prepared by reacting table salt with sulfuric acid, while hydrofluoric acid is prepared from fluorite and sulfuric acid.
Under certain conditions, neutral oxides of the halogens can be isolated from minerals. Examples include Cl2O, Cl2O3, ClO2, Cl2O4, Cl2O6, and Cl2O7. Cl2O7 which can be obtained by dehydrating perchloric acid, HClO4. These oxides are unstable compounds and they can explode under thermal or physical shock. Some halogen oxides are unstable enough to detonate even at temperatures above -40°C.These acids are purified by boiling HF and HCl out of these solutions. The gas is given off when one of these solutions is heated. It is collected from the reactor and then redissolved in water to give relatively pure samples of the mineral acid.
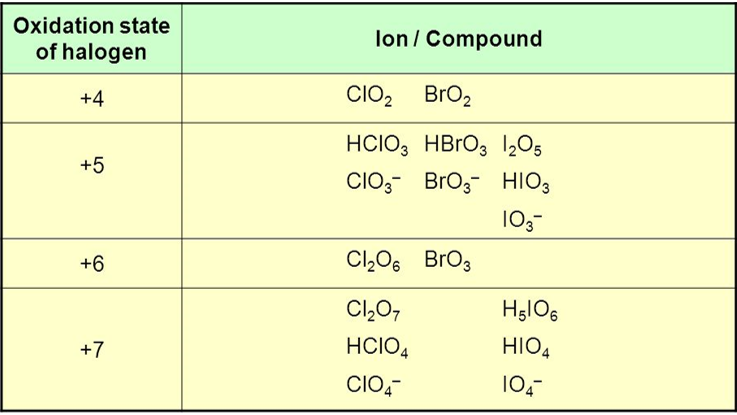
Fig 1: Halogen oxides
Metal halides are compounds of halogens and metals. They include highly ionic compounds, monomeric covalent compounds, and polymeric covalent compounds. Metal halides can be obtained through a direct combination or through neutralization of a basic metal salt with a hydrohalic acid.
Organic halides are organic compounds containing halogen atoms. In the human body, some halogens perform multiple regulatory functions, while others are not essential. Organohalogens are synthesized through the nucleophilic abstraction reaction.
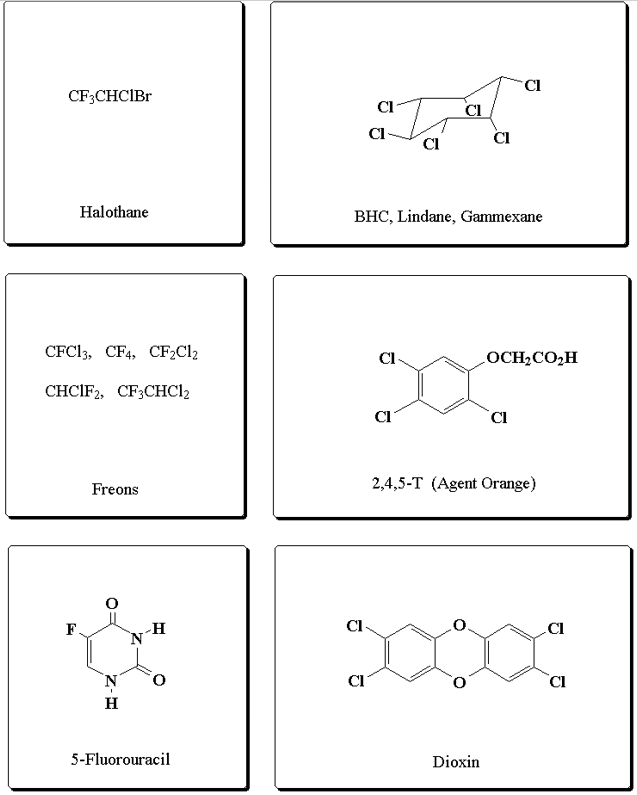
Fig 2: Examples of organic halogen compounds
Compounds substituted with multiple halogens are called polyhalogenated compounds. Many of them are very toxic and bioaccumulate in humans, but they have many potential applications.
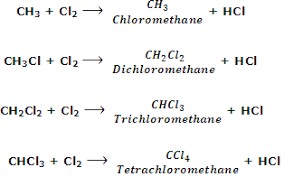
Fig 3: Conversion of methane to polychloro compound.
