The behavior of elements that surround us could be strange and surprising all at the same time. Understanding the properties along with their electronic configurations is an interesting and important concept to observe. Knowing the uniqueness of these properties for specific elements helps a lot in deriving practical applications of them. Not only chemical but these elements also display a lot of physical properties that dictate a lot about them. The study of hybridization is one among them in the realm of science.
What is Hybridization?
The concept of hybridistion was introduced in the year 1931 by Scientist Pauling. According to him it is the redistribution of the energy of orbitals of specific atoms to bring up new orbitals with equal energy. During this process, new orbitals are formed and these are called hybrid orbitals. This process probably includes mixing up and recasting of orbitals.
Steps determining hybridization
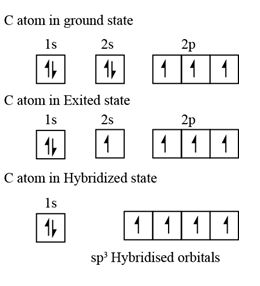
Step 1: The formation of the excited state
- The atom which would initially be in the grounded state will require some energy to sets itself in the excited state. During this process, generally the pair of electrons from the lower energy orbital is known to split. One of the electrons from this is said to be transferred to the slightly higher, empty but the one with almost the same energy. Thus, in the excited state, atoms tend to have more number of orbits with half filled electrons.
- The new number of half-filled orbitals determines how many covalent bonds could be formed for an atom.
Step 2: Mixing and recasting of atomic orbitals
- Now, the orbitals being in an excited state and carrying almost same energy, unite and redistribute electrons (hybridize) to produce hybrid orbitals that possess definite orientation and equivalent energy in space. This mixing and recasting of orbitals with almost similar energy for forming orbitals with equivalent energy, definite orientation and maximum symmetry has been termed as hybridization.
- For instance 2-s and three p orbitals would be mixed to be recasted into four sp3 orbitals.
Step 3: Orientation of the hybrid orbitals
- The hybrid orbitals are further arranged in a way in which mutual repulsion could be minimized. Each one of the hybrid orbitals are known to be concentrated one either one of the sides of the atomic nucleus. Because of this, stronger bonds are formed and they are able to attain greater overlap.
- Thus in case of carbon, the four sp3 hybrid orbitals is arranged at the four corners of the tetrahedron with a view to minimizing mutual repulsion.
Essential conditions for Hybridisation
The orbitals that would be participating in hybridization are required to carry almost the same energy. Thus during the formation of methane, the 2s and 2p orbitals may hold on to relatively similar energy only then it would be possible to recast the orbitals and attain hybrid orbitals. However, hybridization between 2s and 3p may not be possible due to considerable difference between the two.
Apart from the rules and requirements that may prevail in common for hybridization, there are several different types of hybridization that could be studied.
