The mean free path λ of a gas molecule is the normal path length which is between collisions and is given by,
λ = 12√πd2NV
Let’s look at the motion of a gas molecule inside an ideal gas, a typical molecule inside an ideal gas will abruptly change its direction and speed as it collides elastically with other molecules of the same gas. Though between the collisions the molecule will move in a straight line at some constant speed, this is applicable for all the molecules in the gas.It is troublesome to measure or describe this random motion of gas molecules therefore we plan to measure its mean free path λ.
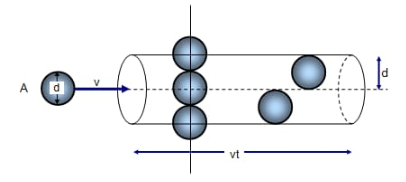
As its name say λ is the average distance traveled by any molecule between collisions, we expect λ to vary inversely with N/V, which is number of molecules per unit volume or the density of molecules, because if there are more molecules more are the chances of them colliding with each other hence reducing the mean free path, and also λ would be inversely proportional to the diameter d of the molecules, because if the molecules were point masses then they would never collide with each other, thus larger the molecule smaller the mean free path, and it should be proportional to π times square of the diameter and not the diameter itself because we consider the circular cross section and not the diameter itself.
Calculations
In reality, the mean free path cannot be calculated by taking the average of all the paths because it is impossible to know the distance of each path traveled by a molecule. However, we are able to calculate it from the average speed (⟨c⟩) of the molecule divided by the collision frequency (Z).
Factors affecting mean free path
Density: As gas density will increase, the molecules become closer to each other. Therefore, they’re additional probably to run into one another, so the mean free path decreases.
Increasing the number of molecules or decreasing the volume causes density to increase. This decreases the mean free path.
Radius of molecule: Increasing the radii of the molecules often decreases the area between them, forcing them to run into each other more often. Therefore, the mean free path decreases.
Pressure, temperature, and different factors that have an effect on density will indirectly have an effect on mean free path.
