In Chemistry, the term “orbital” refers to a function which describes the behavioral pattern of electrons in an atom or atomic nucleus. The term is coined out of the root word ‘orbit’ which describes a circle or something circular. However, in chemistry, the probability of the location of an electron may be dumbbell-shaped, spherical or more complicated shapes of a dumbshell. It was first coined by scientist Robert Mulliken in 1932. However, prior to this period, a scientist,Neil Bohr argued against the notion that electrons can recur around a nucleus with angular momentum, while Hantaro Nagaoka in 1904, brought about the concept of quantum physics in explaining the behavioural pattern of electrons.
Electrons in this context are atomic particles found around an atom’s nucleus. The main purpose of this function is to determine the probability of the location of an electron in an atom. An orbital may also be described as electrons having an energy level designated by special set of numbers called “quantum numbers”. These numbers represent the properties of electrons related to the orbitals. Examples of such numbers are: 1s, 2p, 3d, 4f.
Basically, there are four types of orbitals namely: s, p, d and f. These letters associate orbitald with quantum numbers 0,1,2 and 3 respectively. Thus, while s=0, p=1, d=2 and f=3. The letters stand for alkali metallic spectroscopic lines such as sharp, principal, diffuse and fundamental. Atomic orbitals form the bedrock of electron clouds, which is a framework for determining the behavioural pattern of electrons in a matter.
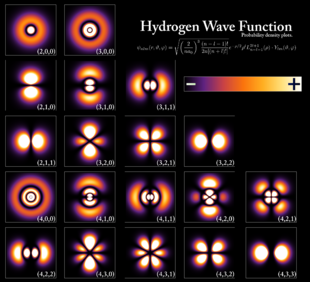
The above diagram explains the atomic orbitals of electrons at different energy levels in a particular atom.
Types of orbitals
1. The s orbital
The s orbital is spherical in shape and can contain a maximum of two electrons, since there is only one available orbital. This orbital increases in size as the energy level in it increases. The following changes take place when the energy level in an s orbital increases:
- They multiply in size,and keep extend to a certain distance from the nucleus
- They bear more nodes
- In a given atom, the s orbitals increase their energy due to the distance from the nucleus.
2. The p orbitals
Just as with the s orbitals, the size of the p orbitals also increase as the major quantum number or energy level increases. However, the shapes of the nodal surfaces of the higher p orbitals remain the same, as illustrated in the diagram below:
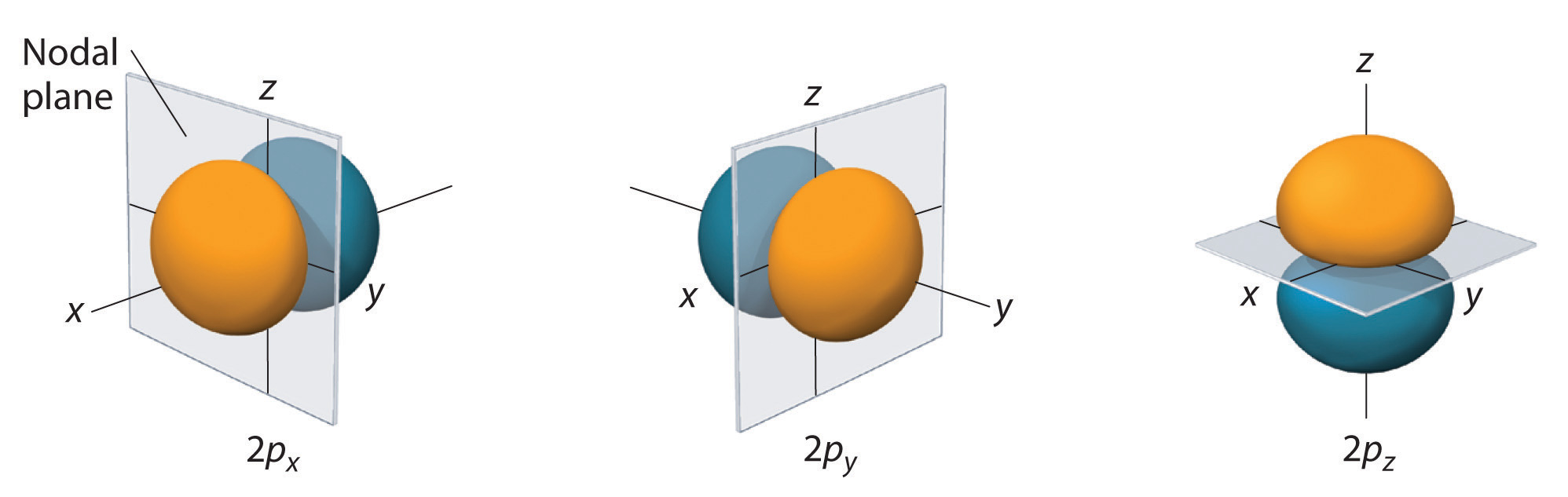
Also, p orbitals are commonly found at the second energy level in addition to the 2s orbitals. Unlike the s orbital, the p orbitals point towards a particular direction as illustrated above.
3. D orbitals
The d orbitals are usually found at the third energy level. This orbital is capable of holding up to 10 electrons, since there are only three orbitals in the shell. The shape of this orbital is twice as complicated as the p orbital, taking the shape of a dumbbell.
