The specific conductance or the conductivity of the electrolyte solution is defined as the measurement of its capacity to conduct the electricity. Conductivity’s SI unit is (S/m) i.e. siemens per metre.
The measurements of conductivity are regularly used in many environmental and the industrial applications as a reliable, inexpensive, and fast way to measure an ionic content in the solution.
Conductivity’s SI Unit is S/m and, unless otherwise certified, it indicates 25°C. The industry often come across traditional unit of μS/cm. = 1 S/cm = 10 to the power 6 μS/cm = 10 to the power 3 mS. μS/cm having the values are lesser than those in μS/m by the factor of 100 (i.e., 100 μS/m = 1 μS/cm).
Occasionally comes across is the moh (which is reciprocal of ohm): 1 mho/m = 1 S/m. As per the history, for several decades, mhos predate Siemens; For example, good testers of vacuum-tube gave the readings of transconductance in micromhos.
R, resistance is proportional to ‘l’ distance, in between electrodes and which is inversely proportional to sample’s cross-sectional region, which is denoted by A. And rho ‘ρ’ is for specific resistance.
R = I / A * p
Practically, cell conductivity is measured by the use of solutions of given ρ*, specific resistance, so the A and I quantities don’t need to be precisely known
R* = C*P*
κ (kappa), the specific conductance is known to be specific resistance’s reciprocal
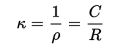
Specific conductance (K) = Conductance = Equivalent conductance ( λ)
If solution is diluted at (9 mL) (9 cm3), solution’s conductance will be identical but the specific conductance is equal to 1/9th of the ratio as it has 9 cubes.
Also, conductance is the same as the equivalent as the solution however contains 1 g electrolyte’s equivalent.
Equivalent conductance “λ” = 9 * k
Generally,
λ = k × V
Where,
V = volume in mL which contains 1 g electrolyte’s equivalent
Factors which affects Electrolytic conduction
Following are the factors affecting Electrolytic conduction level:
- Electrolyte’s essence: The composition and strength of the substance to a great extent affects conduction degree which can occur via the substance.
- Ion’s size is produced and their preservation: Ion’s size in a substance also affects disassociation degree occurring in the substance. Therefore, the properties of salvation influence their conduction.
- Solvent’s nature and its viscosity: Solvent nature and its density even affect substance’s capability allowing to pass the conduction through it.
- Solution’s concentration: The strength in addition to the density of the solution can affect the properties of conduction of the substance.
- Temperature: The highly susceptible conduction increases or decreases under the temperature’s domination of the given substance. On the flip side, conduction in electronic and metallic substance is impacted by the type and nature of metal, substance’ temperature, and the valence electrons’ number present per atom.
