Calorimetry is used for the determination of the heat flow in the chemical reactions, for measurement of the heat of solvation, and characterization of the phase transitions. Generally, the gasses have the two specific heats of the Cp and Cv, at the constant volume and the constant pressure, whereas the solids and liquids have the only single value of the specific heat. But at the higher temperatures, the difference between the Cp and Cv values is not much small.
Description of Calorimetry
Generally, the calorimetry is used for measuring the enthalpy changes during the chemical reactions and processes, where the magnitude of the temperature changes is dependent on the amount of heat that is absorbed or released on the heat capacity of the system. The devices known as the calorimeters are used in the calorimetry which measures the changes in the temperature during the chemical reactions. The thermal energy cannot be easily measured but the temperature changes which are caused by the flow of thermal energy between the substances or the objects can be measured. To have any meaning, the quantity that is measured by following the calorimetric experiment, the changes in the temperature of the device should be related to the heat consumed or evolved in the reaction.
Constant Volume Calorimetry
The constant volume calorimeters such as the bomb calorimeters are used for the measurement of the heat of combustion of the reactions. The bomb calorimetry is used for the measurement of the heat that is absorbed or released by the reaction. The bomb calorimeter is one of the types of constant-volume calorimeter that is used to measure the heat of combustion of the particular reaction.
Constant Pressure Calorimetry
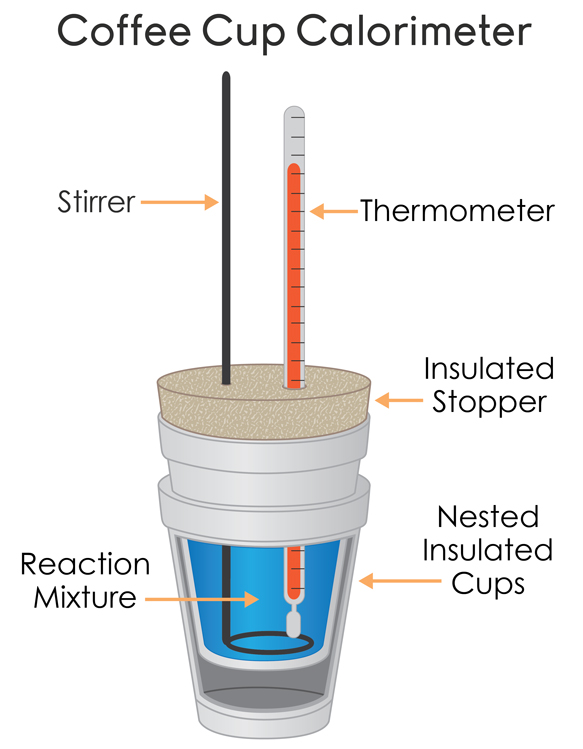
The constant pressure calorimeter is used for the measurement of the changes in the enthalpy of the reaction at the constant pressure in the liquid solutions. One of the simplest examples, of the constant pressure calorimeter, is the coffee-cup calorimeter which is constructed by using the two nested Styrofoam cups and a lid having the two holes which allow the insertion of stirring rod and thermometer. In the inner cup a known amount of the liquid is placed, usually the water for absorbing the heat from the reaction. It is assumed that the outer cup is perfectly adiabatic and there will be no absorption of heat by the outer cup. As such it is assumed that the outer cup is the perfect insulator.
