Depression of Freezing point is a colligative property seen in the solution that is the result from solute molecules’ introduction to the solvent. Solutions’ freezing points are lesser comparative to pure solvent and is proportional directly to solute’s molality.
ΔTf = Tf (solvent) − Tf (solution) = Kf × m ………(1)
ΔTf = Tf (solvent) − Tf(solution) = Kf × m
Where,
ΔTf = freezing point depression,
Tf (solution) = freezing point of the solution,
Tf (solvent) = freezing point of the solvent,
Kf = freezing point depression constant,
m = molality
Major Points Regarding Depression of Freezing Point
- The depression in freezing point can be estimated by the use of the freezing point of pure solvent and solution’s molality.
- At point of freezing, vapor pressure of both liquid and solid forms of the compound should be in equal ratio
- Substance’s freezing point refers to the temperature at which liquid and solid forms are in chemical equilibrium.
- To regain the state of equilibrium, the mixture of solvents’ and solutes’ freezing point is lowered in comparison to the pure solvent
The boiling and freezing point of the pure solvent can be altered when they are added in the solution. When this takes place, the boiling point might result becoming higher while the freezing point result becoming lower of the pure solvent.
These changes can be found using the following formula:
ΔTf =−Kf × m…….(2)
ΔTf = Kb × m………(3)
Where,
m = solute molality,
K = proportionality constants; (Kf = freezing constant and Kb = boiling constant)
To solve for proportionality constant is not the only aim of the query, these values will be given probably. Some general value for Kb and Kf respectively, are
| Solvent | Kf | Kb |
| Water | 1.86 | .512 |
| Acetic acid | 3.90 | 3.07 |
| Benzene | 5.12 | 2.53 |
| Phenol | 7.27 | 3.56 |
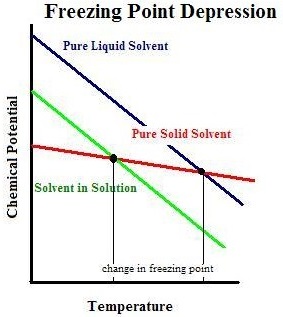
When the freezing point is reached, the chemical capacity of the liquid of pure solvent achieves solvent of pure solid in nature. Repeatedly, since we are working with reduced chemical potential with mixtures, it’s expected the freezing point to vary. On the contrary, the boiling point and the chemical potential of solvent which is impure needs a cooler temperature for it to achieve chemical potential of pure solvent, solid in nature. Thus, depression of freezing point is seen.
Applications
To reduce the freezing point of snow of any place, the road salt takes the advantage of this consequence. When the freezing point decreases, it allows the road ice to melt at low temperatures. The maximum freezing point’s depression is approximately −18 °C (0 °F), therefore, if the ambient temperature is lesser,
NaCl will be inefficient. Under these circumstances,
CaCl2 can in use since it gets dissolved in making 3 ions rather than 2 for NaCl
