Electrolytic Cells
Voltaic cells are operated by the spontaneous chemical reaction, which produces the electrical current through the external circuit.
These cells are significant as they are the base for batteries which boost modern society. However, they don’t exist as the only type of electrochemical cell. In each case, the reverse reaction is non-spontaneous plus it needs the electrical energy.
Introduction
The general form of the reaction can be written as:
Reactants ⇌ Products+Electrical Energy
Spontaneous⟶
⟵Non spontaneous
⟵No
It’s likely to build a cell which works on the basis of the chemical system by running the electric current via the system. These cells are known to be the electrolytic cells.
Electrolytic cells, such as galvanic cells, are comprised of 2 half-cells, one is the reduction in half-cell and the other is the oxidation half-cell.
The electron flow’s direction in the electrolytic cells, nevertheless, can be reversed from the electron flow direction, spontaneous in nature in the galvanic cells. However, the definition of anode and cathode both remains exactly the same, where the oxidation process takes place at the anode and reduction process occurs at the cathode.
As the directions belonging to both the half-reactions are reversed, not magnitude, but the sign of the “cell potential” is reversed.
The electrolytic cells are identical to galvanic (voltaic) cells, in a way that both galvanic and electrolytic cells need a salt bridge, as both are having the anode and cathode side, and both are having a regular electron’ flow to the cathode from the anode.
Nonetheless, there exist the noticeable differences in between two cells. The primary difference outlined below is:
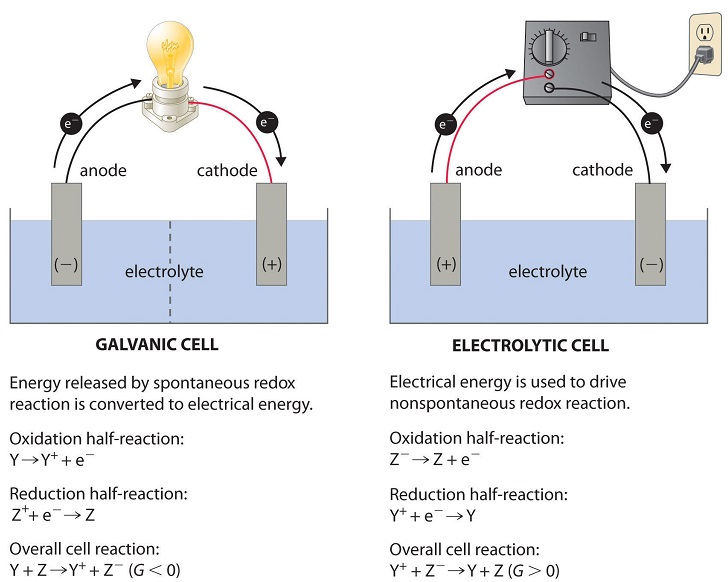
Fig 1
| The Electrochemical cell (Galvanic Cell) | Electrolytic cell |
| The Galvanic cell converts chemical energy into electrical energy. | The electrolytic cell converts electrical energy into chemical energy. |
| Here, the redox reaction is responsible and for electrical energy production and the reaction is spontaneous. | The redox reaction is not in spontaneous nature and the electrical energy is supplied to start the reaction. |
| 2 half-cells are arranged in various containers; they are connected via porous partition or salt bridge. | Both electrodes are arranged in the exact same container in a solution of liquified or molten electrolyte. |
| The reaction taking place at the cathode is called the positive electrode whereas the anode is the negative electrode. | In the electrolytic cell, the cathode is a negative electrode, on the other hand, the anode is positive. The reaction taking place in the cathode is the reduction reaction and at the anode, it’s the oxidation reaction. |
| The supply of electrons is by oxidizing species. Their movement is from anode to cathode in the outer circuit. | The battery present externally supplies electrons. They penetrate through cathode section and come out via anode. |
