Einstein’s photoelectric effect- particle nature of light
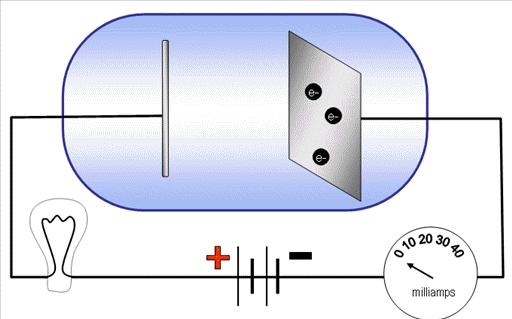
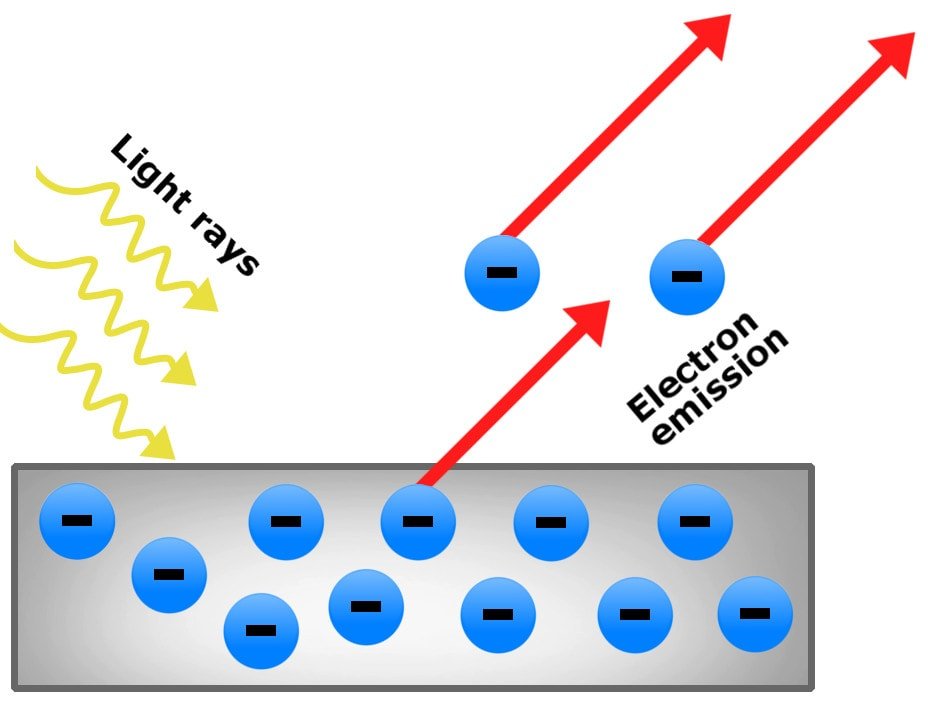
Albert Einstein great scientist in 1905 proposed that light can be called quanta of energy that also behalf as particles. He also explained that photon is a particle of electromagnetic radiation that has zero mass and also carries a quantum of energy. This is tried and tested because for many years light has been described using only wave concept. The photoelectric effect is a phenomenon that can be seen when the light shined onto the metal surface which causes the ejection of electrons from the metal. If classic physics are taken into consideration then it is impossible to explain the photoelectric effect and if this classic physics can be applied in this effect it can generate enough energy for electrons to be ejected from the surface and this can also happen even if the light is at low frequency.
What lead to the study of particle nature of light to be experimented?
Einstein mentioned that the photons of the light beam have a characteristic of energy which is somehow proportional to the frequency of light. A condition is applied if the energy of the photon is too low the electron is unable to escape the material. This is mentioned that the energy of the emitted electrons doesn’t depend on the intensity of the light which is coming in but can only affect the individual photons only and it is an incident of an interaction between the incident photons and innermost electrons. It is definite that photoemission can occur by any material but mostly it is observed from metals or other conductors as the process produces a charge imbalance and this is meant that these are not charged by current flow. One more interesting fact is that when a photoelectron is emitted into solid rather than vacuum a term is used known as internal photoemission.
What are the three-step models?
It is mainly used in X rays to be crystalline and often decomposed in three steps as the first step is the inner photoelectric effect in which the left hole can give rise to auger effect which can be visible even when the electron stays there. Second is ballistic transport which is half of the electrons to the surface but some of the electrons scattered. The third is the electrons escape of the materials from the surface. These three are the three-step models of photoemission which was advanced by Einstein by putting his extra efforts into this concept.
