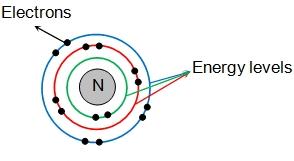
The energy level in quantum mechanics defined as a system or particle that is bond and confined spatially or which can only take on certain discrete values of energy. This makes a contrast between this and classical particles which can have any amount of energy. In physics, this term is commonly used for the energy level of electrons, atoms, ions and molecules which are mainly bound by an electric field. It is mentioned that if the potential energy is set to zero at a distance of infinite from atomic nucleus for the usual convection which has negative potential energy. And if an atom, ion or molecule are the lowest possible energy level then it is said to be ground state. Each shell can have a definite amount of electrons as first shell can have only two electrons, second can have eight and third can have eighteen and so on with every shell.
How energy work in an atom?
In many explanations and theories, it is said that if the energy of an electron at a various level is lower than in an atom the zero points for energy are set. If we take an example of one electron in an atomic orbital in a hydrogen-like an atom or can be called ion then it states is mainly determined by the interaction of negative electrons and positive electron. But there is a condition if there is more than one electron around the atom then electron-electron interaction will raise the energy level and if the wave function is low then these interactions are often neglected. Structures like fine structures arises from relativistic kinetic energy and if more elaborated then it is an electrodynamic interaction between the electron spin and motion and the electric field of nucleus. There is a hyperfine structure also due to electron nucleus spin-spin interaction which results in a typical change in energy.
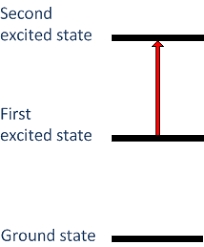
Energy in the molecules and transition?
A chemical bond formed in atoms in the molecules because they can make any situation more stable with the involved atoms which means the sum energy levels are involved atoms in molecules is lower. Another important factor of energy is the transition as electrons in atoms and molecules can change level of energy by emitting or absorbing a photon which is effectively moving electrons out of an orbital with the principle of quantum number which may have a practical effect on the remaining atoms.
