Surface Chemistry: Enzyme catalysis and colloidal state
Enzymes are extremely effective biologically active catalysts. They are homogeneous catalysts, reacting in solution with body fluids. Only one type of molecule selectively fits the active site in its “lock and key” mechanism. Therefore, enzymes are very specific as to what they catalyse on a specific substrate. Enzymes are typically large protein molecules but only a small part of the molecules actsually involved in the reaction. The active site has two basic components called catalytic site and binding site.
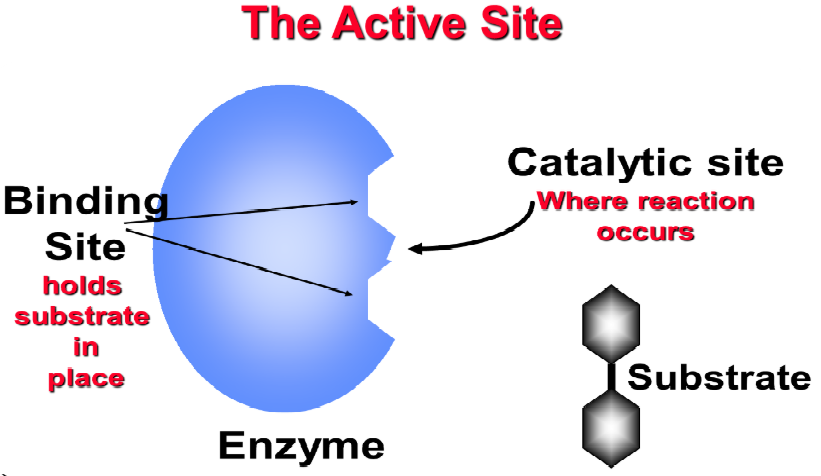
Fig 1: Components of an enzyme.
Enzyme catalysis is the process in which the increase in the rate of a chemical reaction takes place by using the active site of a protein. The protein catalyst(enzyme) may be part of a multi-subunit complex, and/or may transiently or permanently associate with Cofactor (e.g.adenosine triphosphate). Catalysis of biochemical reactions in the living cell is vital because of very low reaction rates of the uncatalysed reactions.
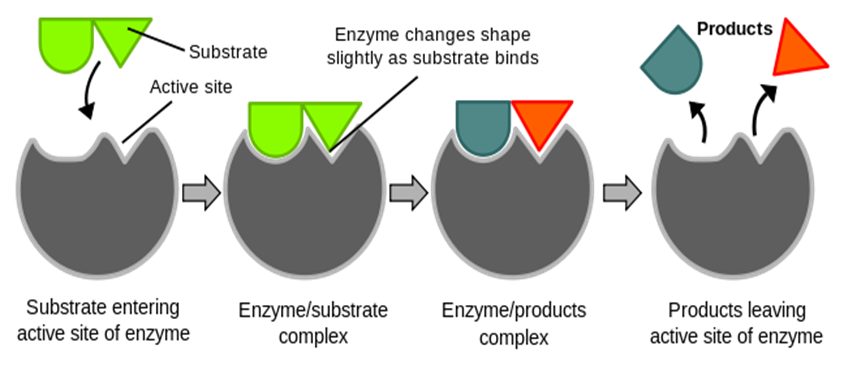
Fig 2: First the enzyme and substrate combine to form a complex. Next, an enzyme-product complex is formed.
The distinction between true solutions, colloids and suspension
A solution can contain two types of soluble substances: crystalloids and colloids. Solutes obtained in crystalline form and can easily dissolve in a solution that passes through a membrane are crystalloids. Examples include salt, sugar, urea etc. Alternatively, colloids show little to no tendency to diffuse through a membrane when in solution. This substance cannot be separated from the solvent using the ordinary filtration process.
| True Solution | Colloid | Suspension |
| A solution is a homogeneous mixture. Only soluble substance form a true solution | A colloid is a heterogeneous mixture containing particles. The solute particles are at an intermediate state between true solution and a suspension. | A suspension is a heterogeneous mixture from which particles settle out upon standing. At least two substances can be clearly identified in a suspension. |
| Solution particles are smaller than 10-7cm or 1 nm | Colloids have particles smaller than those in suspensions and larger than those in solutions and range in size from 1 nm to 1000 nm. | Suspension contains large and visible solute particles> 1000 nm |
| Metal alloy: solid-solid solution. Saltwater: Solution of solid, NaCl and liquid, water. Vinegar: Liquid-liquid solution of acetic acid and water. Air: Solution of several gases Coke: Gas-Liquid solution of CO2 in water. | The particles are spread, or dispersed, throughout the dispersion medium, which can be a solid, liquid, or gas. Examples include soap solution, starch solution, milk, ink, jelly and detergent. | Chalk water, muddy water and sand in water are examples of solid-liquid suspension. |
| They do not settle out with time and remain stable. | They do not settle out with time. | They do not stay suspended indefinitely and the suspension is unstable. |
| True solutions will allow light to pass freely through it without any interference. No Tyndall effect | Colloids allow light to pass through, but not without some scattering. Tyndall effect is applicable | Suspensions prevent light from passing through them. Tyndall effect is not applicable |
| No Brownian movement | Browning movement | The system needs constant stirring. |
 Fig 3: The small size of the solute particles in a solution allows them to pass through filter paper. |  Fig 4: The intermediate sized particles cannot be retained by filter paper. |  Fig 5: The particles of a suspension can be removed by filtration. |
