Surface Chemistry: Factors affecting the adsorption
Nature and surface area of the adsorbent: Activation of adsorbent is necessary to provide vacant sites on its surface for the adsorbate to bind. The solid crystals need to be broken down to form small particles or powdery form to increase in surface area for binding. Most of the surface area is occupied with pores of molecular-sized dimensions. This results in slower mass transfer during the time period in which the adsorption process takes place. However, it also facilitates the greater binding capacity of the adsorbate. Accordingly, smaller particle size reduces the internal diffusional and mass transfer limitations to the penetration of the adsorbate into the adsorbent. Therefore, the equilibrium between adsorption and desorption is more easily achieved where almost complete adsorption capacity of adsorbate can be occupied by the adsorbent. Similarly, easily liquefiable gases get easily adsorbed by physisorption
Nature of adsorbate: In general, as the solubility of the solute increases the extent of adsorption decreases. This is known as the “Lundelius’ Rule”. Competition between the preference of solute-solid surface binding with solute-solvent attraction takes place. Adsorption of a solute from dilute solution involves breaking solute solvent bond and adsorbent solvent bond along with the formation of an adsorbent solute bond. Variables that affect solubility include ionization, polarity and molecular size. Solubility is minimum when compounds are uncharged. As polarity increases, the adsorbate gets higher solubility because water is a polar solvent) while high MW of adsorbate decreases the solubility.
Temperature: physisorption occurs at low temperature while chemisorption increases with temperature. Initially, a higher temperature is required to overcome the activation energy, followed by a decrease in adsorption due to heat release. Adsorption reactions are almost always exothermic i.e., ∆Hrxn is generally negative. Here heat is given off by the reaction therefore as T increases the extent of adsorption decreases. The adsorption capacity of the adsorbent decreases with increasing temperature.
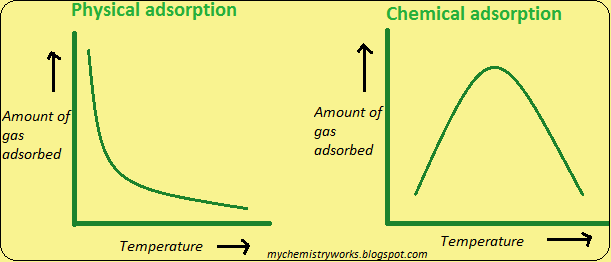
Fig 1: Relationship between adsorption and temperature
Pressure: It shows its effect on the adsorption of gases where the partial pressure of gases increases with increasing pressure at a constant temperature. The partial pressure of different types of gas molecules is proportional to the total pressure. However, change in temperature may decrease the rate of adsorption. Likewise, the increase in adsorption only occurs up to a saturation point after which the no adsorption takes place regardless of any externally applied pressure.
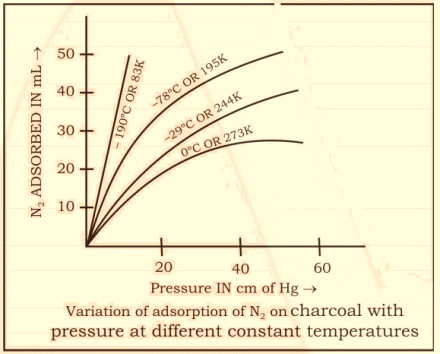
Fig 2: Variation in the adsorption of nitrogen gas on charcoal with variable pressure and temperature.
Contact time of adsorbent and adsorbate: Longer time enables the adsorption to move towards completion.
Presence of foreign cations and anions: In general, get competition for a limited number of sites, therefore, get the reduced extent of adsorption or a specific material. Removal of adsorbed impurities increases the amount of adsorption.
Nature of medium: Adsorption of the solute will be at maximum if the solvent is inert. Therefore, a medium that has no affinity for adsorbent or for the solute.
The pH of the adsorption medium: pH often affects the surface charge on the adsorbent as well as the charge on the solute. However, change in adsorption with pH is associated with ionization efficiency of the adsorbent and adsorbate molecules and their intrinsic nature. Generally, the pH of organic material goes down as the adsorption magnitude goes up.
