Molecular shape
In molecular shape (the shape of a single molecule), it is important to determine how there are the interaction and reaction process of molecules with each other or interaction and reaction amongst themselves.
Also, molecular shape affects the melting and boiling point of the molecules. Let suppose, if all the molecules were linear, then life wouldn’t have existed.
Several properties of molecules originate from specific shapes, which a molecule consist of. Let’s say, if the water(H2O) molecule was linear, then it would have been non-polar and therefore, it would not consist of all the special properties it is containing.
Determining molecular shape
To envision the covalent molecule’s shape, pursue these steps:
- Sketch the diagram of a molecule with the use of the Lewis diagram. Assure that you notify all the valence electrons surrounding the central atom of the molecule.
- Calculate the number of electron pairs all over the central atom.
- Using the below-mentioned table, find out the basic/original geometry of the molecule. Let’s say, a molecule having 2 electron pairs and without lone pairs all over the central atom consist of a linear shape, and the other molecule having 4 electron pairs without having lone pairs surrounding the central atom will result having a tetrahedral shape.
| Number of bonding electron pairs | Number of lone pairs | Geometry | General formula |
| 1 or 2 | 0 | linear | AX or AX2 |
| 2 | 2 | bent or angular | AX2 E2 |
| 3 | 0 | trigonal planar | AX3 |
| 3 | 1 | trigonal pyramidal | AX3E |
| 4 | 0 | tetrahedral | AX4 |
| 5 | 0 | trigonal bipyramidal | AX5 |
| 6 | 0 | octahedral | AX6 |
Table 1: The consequence of electron pairs to determine the molecules’ shape.
“A” represents the central atom and “X” are the terminal atoms.
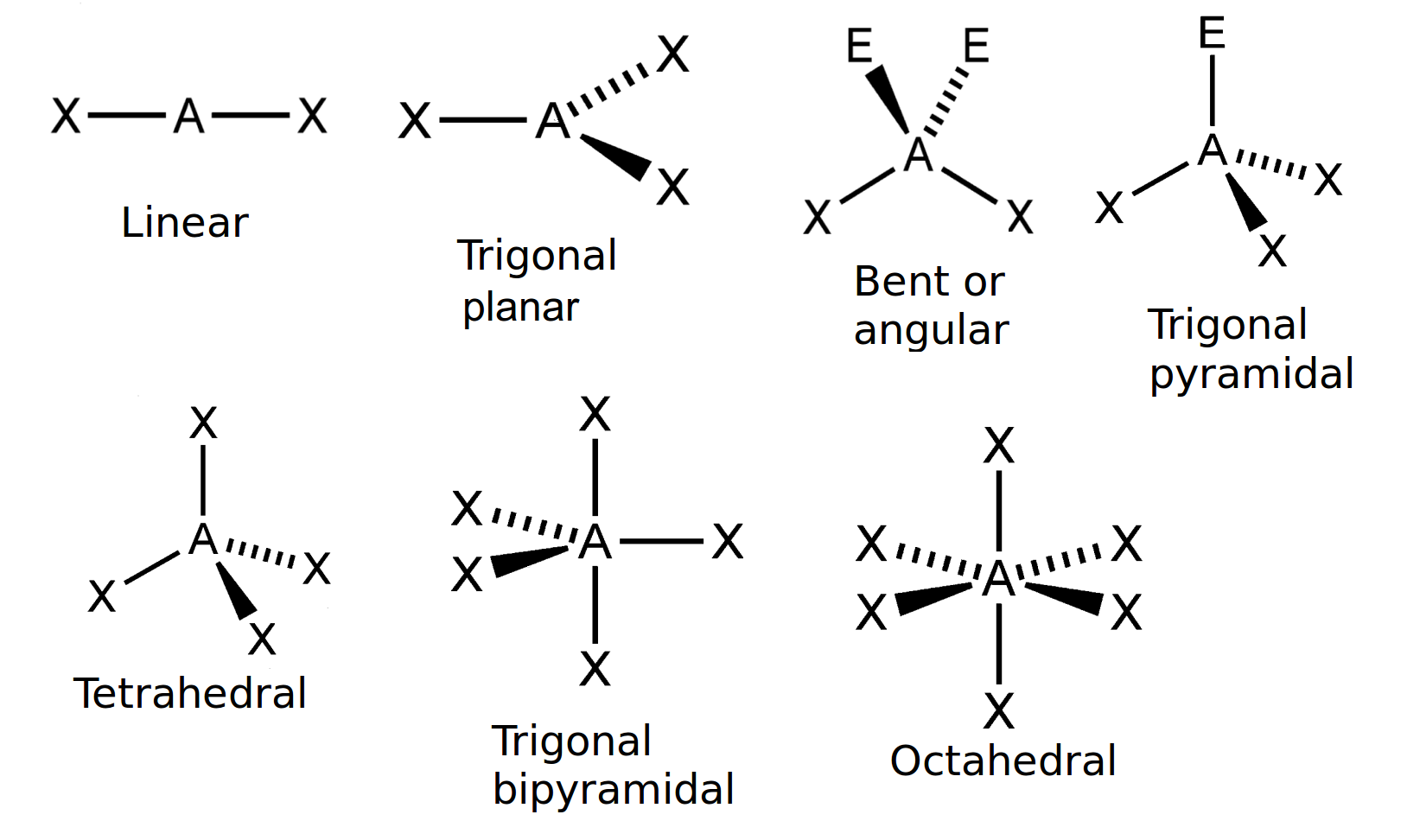
Figure 1: The simple molecular shapes.
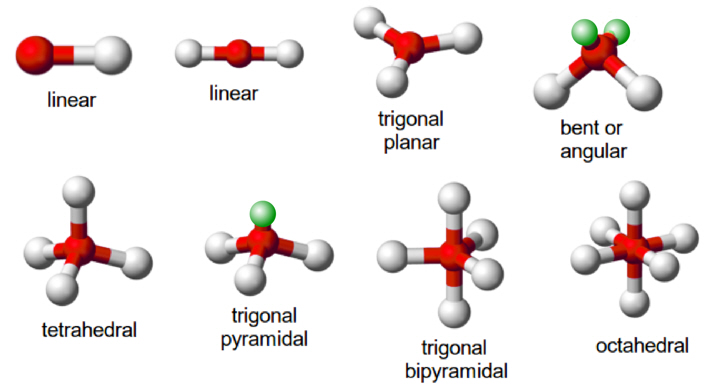
Figure 2: The common molecular shapes in 3-D.
Green ones are the lone pairs; red balls (A) are the central atoms; white balls (X) are the terminal atoms
Valence shell electron pair repulsion (VSEPR) theory
The covalent molecule’s shape can be estimated using the “Valence Shell Electron Pair Repulsion (VSEPR)” concept. Simply, the VSEPR theory states that in a molecule, the valence electron pairs will organize themselves surrounding the central atom (s) of a molecule and accordingly the repulsion acting between the negative charges of theirs is as tiny as possible.
Talking another way round, pairs of valence electron organize themselves in a way that they at a far distance as possible.
VSEPR model is not a concept or the theory; It doesn’t make an effort to illustrate observations. Rather, it’s a counting process that precisely predicts the 3-D structures of large no. of compounds, that can’t be predicted to use the “Lewis electron-pair approach.”
The VSEPR model is able to decided the structure of any molecule or polyatomic ion in which the central atom is a nonmetal, the structures of many molecules as well and polyatomic ions with a central metal atom.
The prediction of the VSEPR model can give the estimation of the structure of approximately any polyatomic ion or any molecule in which the central atom is the non-metal along with structures of several polyatomic ions or molecules accompanying a “central metal atom.”
A few of the following things need to be kept in mind in the VSEPR theory:
- To utilize the model of VSEPR, it’s important to estimate the molecular geometries.
- It’s vital to estimate whether the molecule is having a dipole moment.
Summary
The prediction of bond types and the number of bonds is being done by “Lewis electron structures”. Whereas, VSEPR theory can clearly predict the shapes of several polyatomic ions or molecules.
Also, one major point to keep in mind is that in molecular shape, all diatomic compounds are having a linear shape.
