Chemical Thermodynamics: Heat capacity and Specific heat
Heat capacity is the amount of heat(measured in Joules or Calories) required to raise a unit amount of substance (measured in grams or moles) the temperature of an object by 1°C temperature (measured in C or K). The heat capacity of an object is
This addition of energy or heating can be carried out at constant volume or constant pressure. At constant pressure, some of the heat supplied goes into doing work of expansion and less is available with the system (to raise its temperature).
Heat capacity at constant Volume (CV):
It is portrayed as the slope of the plot of internal energy with temperature.
Heat capacity at constant Pressure (CP):
It is portrayed as the slope of the plot of enthalpy with temperature.
Water has a high heat capacity (of CP = 4186 J/K/mole =1 Cal/C/Kg) and it heats up slowly in comparison to air (with heat capacity, CP = 29.07J/K/mole). Consequently, oceans heat up slowly as compared to the atmosphere.
As T – > 0K, the heat capacity tends to zero. Hence, near 0 Kelvin minimal heat is required to raise the temperature of a sample.
Specific heat is defined as the amount of energy required to raise the temperature of 1 gram of a substance by 1°C under standard pressure. Also, the specific heat capacity is the heat capacity per unit mass, . Accordingly, heat capacity can be computed for any combination of conditions such as constant V, constant P, constant P&V or other intensive or extensive properties.
Heat capacity is an intrinsic physical property of a substance. It measures the amount of heat required to change that substance’s temperature by a specific amount. In the International System of Units (SI), heat capacity is denoted as joules per kelvin (J\K). Likewise, heat capacity is also is dependent on the size and the mass of substance under consideration.
There are two derived quantities that specify heat capacity as an intensive property (i.e., independent of the size of a sample) of a substance: the molar heat capacity, which is the heat capacity/mole of a pure substance.
Molar heat capacity is subjected to pressure or volume respectively and it is often designated as CP, to denote heat capacity under constant pressure conditions, as well as CV, to denote heat capacity under constant volume conditions. The specific heat capacity is also called specific heat, which is the heat capacity per unit mass of a pure substance.
Calculating Specific Heat Capacity
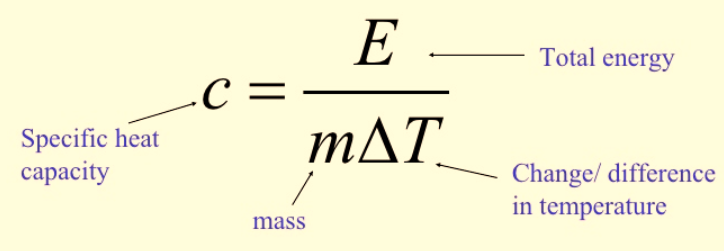
![]()
Figure 1: Calculation of specific heat capacity
Some common specific heat and heat capacities:
| Substance | S (J/g ºC) | C (J/ºC) for 100 g |
| Air | 1.01 | 101 |
| Aluminum | 0.902 | 90.2 |
| Copper | 0.385 | 38.5 |
| Gold | 0.129 | 12.9 |
| Iron | 0.450 | 45.0 |
| Mercury | 0.140 | 14.0 |
| NaCl | 0.864 | 86.4 |
| Ice | 2..03 | 203 |
| Water | 4.179 | 417.9 |
A derivation of heat capacity and specific heat capacity is associated with the quantity of the substance involved in the reaction. Wherein, the amount of substance is expressed as moles ‘µ’ and we can define the heat capacity per mole of a substance by:
