Involving s, p and d orbitals
What do you mean by Hybridization?
To give orbit the equivalent energy, the redistribution of orbital energy of individual atoms occurs when 2 atomic orbitals integrate together for the formation of a hybrid orbital in a single molecule. This process is known as hybridization. In this process, the new orbitals which are thus formed are known to be the hybrid orbitals.
Types of Hybridization
- sp Hybridization
- sp2 Hybridization
- sp3 Hybridization
- sp3d2 Hybridization
- sp3d2 Hybridization
Depending on the types of orbitals which are mixed up in combining hybridization can be categorized as sp3, sp2, sp, sp3d, sp3d2, and sp3d3. Now, let’s discuss different types of hybridization with their examples.
sp Hybridization
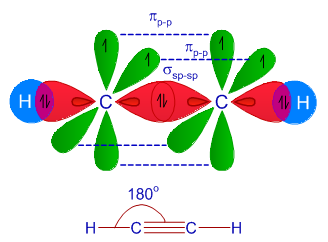
The SP hybridization is seen when orbital of S and P one each in the very same prime shell of the atom combine to form all two new “equivalent orbitals”. The formation of new orbitals is referred to as the sp hybridized orbitals. The SP hybridization forms linear molecules, having a bond angle of 180 degrees.
- In this type of hybridization, there is mixing of one orbital of ‘s’ and one orbital of ‘p’ of equivalent energy to give rise to a new hybrid orbital being referred to as the SP hybridized orbital.
- The other name given to SP hybridization is “diagonal hybridization”.
- Each sp orbital hybridization consist of the same amount of characters of ‘s’ and ‘p’, that is, 50% S and 50% P character.
A few examples of sp Hybridization:
- All Beryllium compounds such as BeH2, BeF2, BeCl2
- All carbon-containing compounds which involve triple bond just like C2H2.
sp2 Hybridization
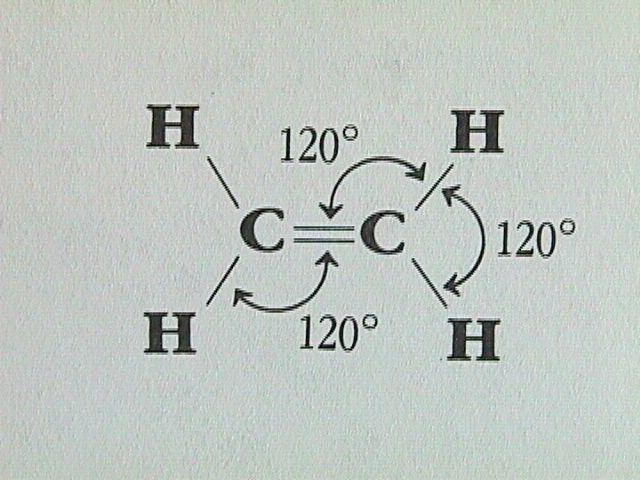
sp2 hybridization is seen when orbitals of single s and two p of the identical shell of the atom are combined for the formation of 3 equal orbitals. Thus, the new orbitals which are formed; known as sp2 hybrid orbitals.
- Another name for sp2 hybridization is “Trigonal hybridization”.
- This hybridization involves the mixing of single ‘s’ and two ‘p’ orbitals of equivalent energy level to deliver an all-new ‘hybrid orbital’ called, sp2.
- In the trigonal symmetry, the orbital mix of s and p formed is managed at an angle of 120 degrees.
- In one single plane only, all 3 hybrid orbitals find their place and create a 120 degrees angle with one another. Every hybrid orbital formed consist of 33.33%‘s’ character and ‘p’ having 66.66% of character.
- The molecules where a central atom is connected to 3 atoms and where there’s sp2 hybridization, they result in triangular or trigonal planer shape.
Examples of sp2 Hybridization
- All Boron compounds like BF3, BH3
- All carbon-containing compounds formed by double bond like Ethylene (C2H4)
sp3 Hybridization
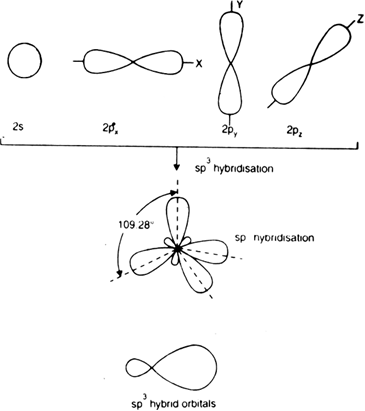
When three ‘p’ and one ‘s’ orbitals of the identical shell of the atom combine for the formation of 4 new equal orbitals, so this hybridization type is known as sp3 or tetrahedral hybridization. The formation of new orbitals is called sp3 hybrid orbitals.
- A regular tetrahedron structure is formed, having 4 corners, making 109°28’ angle with one another.
- sp3 hybrid orbitals’ angle formation is at 109.280
- This hybrid orbital of sp3 consists of 25% character of ‘s’ and 75% character of ‘p’.
Example: Ethane (C2H6), methane.
sp3d Hybridization
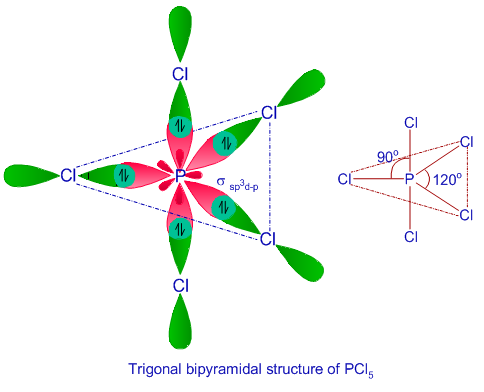
Hybridization of sp3d includes the mixing of orbitals of one ‘s’, 3 ‘p’, and 1 ‘d’ to set up 5 sp3d hybridized orbitals of equivalent energy. They consist of trigonal bipyramidal geometry.
- The combination of orbitals of s, p, and d forms triangular/ trigonal bipyramidal geometry.
- The formation of 3 hybrid orbitals takes place in a horizontal plane, making an angle of 120° with each other called, “equatorial orbitals”.
- The rest of the two orbitals forms 90 degrees angle, lying in a vertical plane of equatorial orbitals called, axial orbitals.
Example: Phosphorus pentachloride (PCl5)
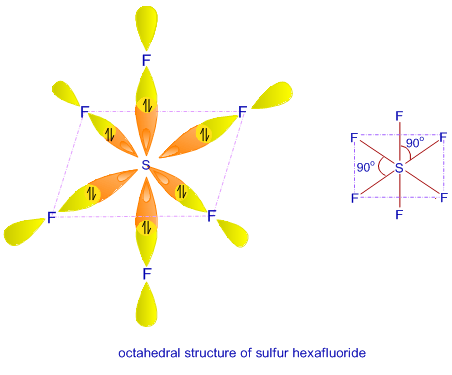
sp3d2 Hybridization
 \
\
- This hybridization contains a mixture of orbitals of 1s, 3p, and 2d, and combine to form six same “sp3d2 hybrid orbitals’.
- These new formed six orbitals are directed towards angles of the octahedron.
- The hybridization is formed at a 90-degree angle to one another.
