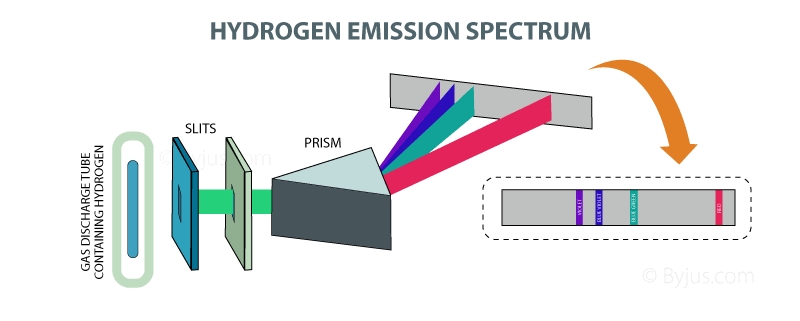
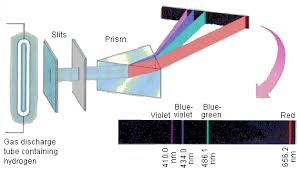
All the lines in the absorption and emission of the spectrum of hydrogen when each one corresponding to an allow transition between the level of quantum energy it has been divided into a number of spectral series with wavelength given and these transitions are been seen between the energy due to the working of an electron in between. It is stated that a hydrogen atom which consists of electron orbiting in the nucleus and the electromagnetic force happening between the electron and the nuclear proton leads to a state of quantum states and these stages were visualized by the Bohr model of the hydrogen atom as being distinct around the orbit of the nucleus but the Bohr model was then replaced by quantum mechanics in which the electrons occupy an atomic orbital. From higher energy state to lower energy state spectral emission occurs when an electron transition, or jumps.
What are the extensions of this spectrum and the physics?
The concept of some scientist can be applied to any system with a single particle which is orbiting the nucleus as this equation must be based on modification that is based on Bohr’s radius by which emissions will be of similar character but at a different range of energies but later it was correct by Bohr’s equation from which spectral lines arise from the molecules. All other atoms possess at least two electrons in their neutral form and the interaction between these electrons which most do the analysis by some sine methods. The formula of the Rydberg was the major step of physics but was so long before an extension of the spectra of other elements it is same as the absorption or the emission of photons by an atom when the conversion law holds for the whole system for example of an atom plus the photon.
What is the series involved in the spectrum?
There are so many series that are involved in the hydrogen spectrum which is namely Lymanseries, Balmer series, Paschen series, brackets series, fund series, Humphrey series and many more are included. Every series has its own equation and its own specification. Lyman series includes those lines emitted by the transition of electrons from an outer orbit of the quantum number and the Balmer series can be seen in the solar spectrum. And there are so many applications and series that are completed and tested to try hydrogen of the spectrum.
