The amines are basically the derivatives of the ammonia. The derivatives of ammonia are formed by replacing the hydrogen atoms of the ammonia. The ammonia having nitrogen and ligand in its orbital behaves as a nucleophile. Thus the ammonia undergoes nucleophilic substitution reaction with the alkyl halides. Thus, with further substitution, the higher order of amines are produced subsequently. The amines are widely used in many industries for making medicines, dyes and refrigerants.
The amines are classified on the basis of the number of the alkyl or the aryl compounds attached to the nitrogen atom. Many tests are there for the identification of different orders of amines. But, from all the tests for the identification of amines the Hinsberg test is the most famous among all. The reactions this produced are called as Hinsberg reactions.
Hinsberg reagent reacts differently and gives different products with all the different degrees of amines, hence it is one of the most famous tests.
Identification of primary amine
Primary amines are the amines in which the nitrogen atom is bonded with one carbon atom, the rest two are hydrogen atoms and a ligand is there on the nitrogen atom. The primary amine is also known as 1° amine. Another name for one degree amine is amino.
The amines react with the Hinsberg reagent. The Hinsberg reagent is benzene sulphonyl chloride (C6H5SO2). The primary amines reacts with the Hinsberg reagent to give the amide as an end product. The amide produced by the reaction of primary amine and benzene sulphonyl chloride is alkyl benzenesulphonyl amide.
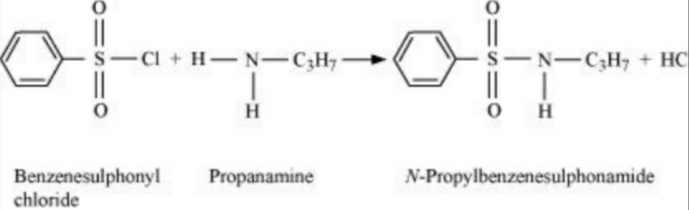
The sulphonyl the group in the Hinsberg reagent is a strong electron withdrawing group. The sulphonyl group, as a result, releases the H proton gets released. Hence, it is basic and dissolves in the alkali.
Identification of secondary amines
The secondary amines are also known as the 2° amines. The secondary amines are formed by the binding of two carbon atoms to the nitrogen atom with the ligand on the nitrogen. The secondary amine consists of one hydrogen atom.
When the Hinsberg reagent, benzene sulphonyl chloride reacts with the secondary amine, it gives an amide as an end product. The sulphonamide thus formed in the end product does not have any hydrogen atom. Hence, the amide is not acidic and hence Hinsberg reagent is insoluble.
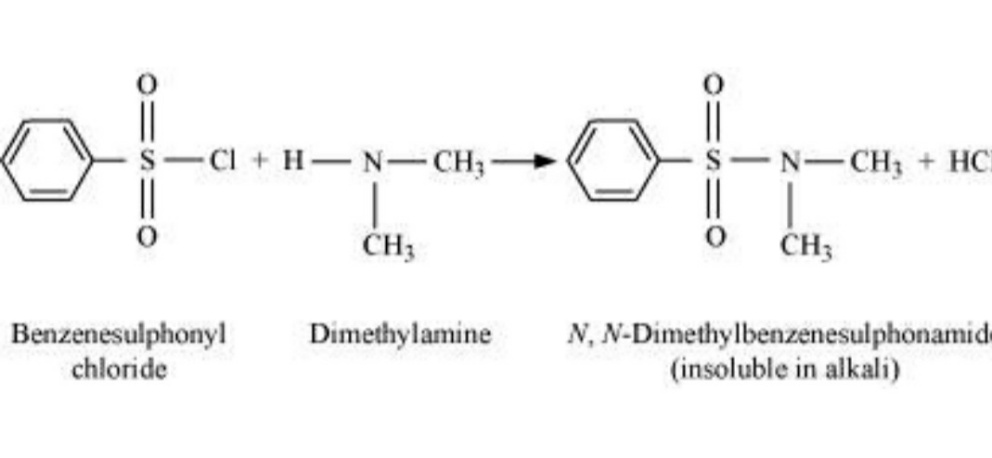
Identification of the tertiary amines
The tertiary amines are the amines in which there are three carbon atoms attached to the nitrogen. Herein, all the hydrogen atoms are replaced by the aryl or the alkyl group. The tertiary amines are also known as 3° amines. Since there are no Hydrogen atom in the tertiary amine, it does not react with Hinsberg reagent.
