p-Block Elements: Coordination compounds
Introduction
The transition metals and their ions have a much higher tendency to form coordination compounds as compared to the s- and p- block elements. The relatively smaller sizes, higher ionic charges and the availability of d orbitals for bond formation allow transition metals to form complex ions. Coordination compounds, unlike normal compounds, retain their structure when dissolved in water or any other suitable solvent. The properties of these compounds are totally different from those of their constituents.
Fig : A central metal atom can bond to a group of molecules or ions: metal complex. If it’s charged: complex ion.Compounds containing complexes are coordination compounds.Complex ions remain intact upon dissolution in water.
Alfred Werner is a pioneer in the field of coordination chemistry for his work in understanding the coordination compounds. He received the Nobel Prize in 1913 in recognition of his efforts. He showed that neutral molecules were bound directly to the metal so that complex salt such as CoCl3.6NH3 is correctly formulated as [Co(NH3)6] 3+(Cl– )3. G.N. Lewis and N.V. Sidgwick proposed that the formation of a chemical bond requires sharing of an electron pair.
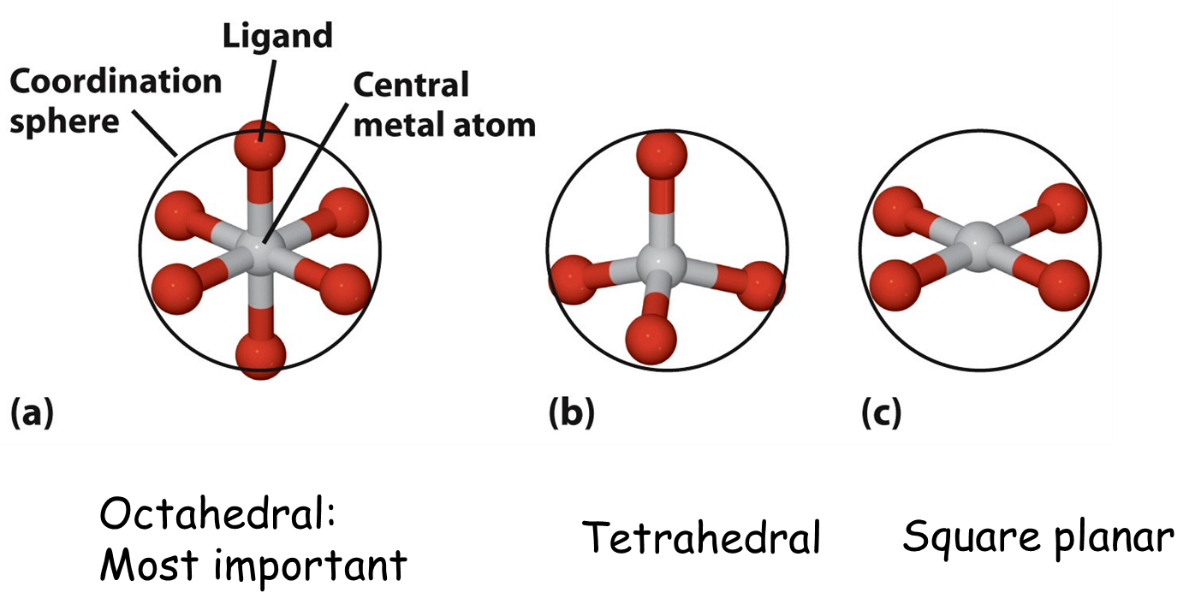
As shown in the figure above, in a coordination compound, the metal species acts as electron acceptor (Lewis acid). The neutral molecule with a lone pair of electrons or anion acts as an electron donor (Lewis bases). A metal atom or ion bonded directly to a fixed number of anions or molecules together form a coordination entity. For example, [Pt(NH3)2Cl2] is a coordination complex with platinum(II) is surrounded by two ammonia molecules and two chloride ions. Other examples are [Fe(CN)6] 4-, [Co(NH3)5Cl]2+, [Ni(CO)4] etc. In a coordination entity, the central atom or ion is the component to which a fixed number of ions/ groups are bound in a definite geometrical arrangement. For example, the central atom in [Ni(NH3)6] 2+ is Ni(II), in [Mo(CN)8] 3- , Mo(V), and in [Co(Pr3)3Cl], Co(I). The charged ions or neutral molecules bound to the central atom in the coordination entity are called ligands. For example, PR3 and Cl- are ligands in [CoCl(Pr3)3]. The number of ligand donor atoms that are directly bonded to the central atom is called the coordination number. For example, consider the complex ions [Mo(CN)8] 3- and [CoCl (Pr3)3].Here, the coordination number of Mo and Co are 8 and 4, respectively. The central atom and the ligands bonded to it are enclosed in the square bracket and is collectively termed as the coordination sphere. Coordination sphere in the complex [Ni(NH3)6]Cl2 is [Ni(NH3)6] 2+. Coordination polyhedron is the spatial arrangement of the ligands around the central atom. The most common coordination polyhedra are octahedral, tetrahedral and square planar as shown in the figure below.