d and f Block Elements
General Introduction
Transition metals are divided into two forms—transition metals and inner transition metals. They are classified based on their electron configurations. In the case of a transition metal, the highest occupied s sublevel where a nearby d sublevel contains electrons giving then another name – d block. Alternatively, in atoms of an inner transition metal, the highest occupied s sublevel while a nearby f sublevel mostly contains electrons giving them another name – f block.
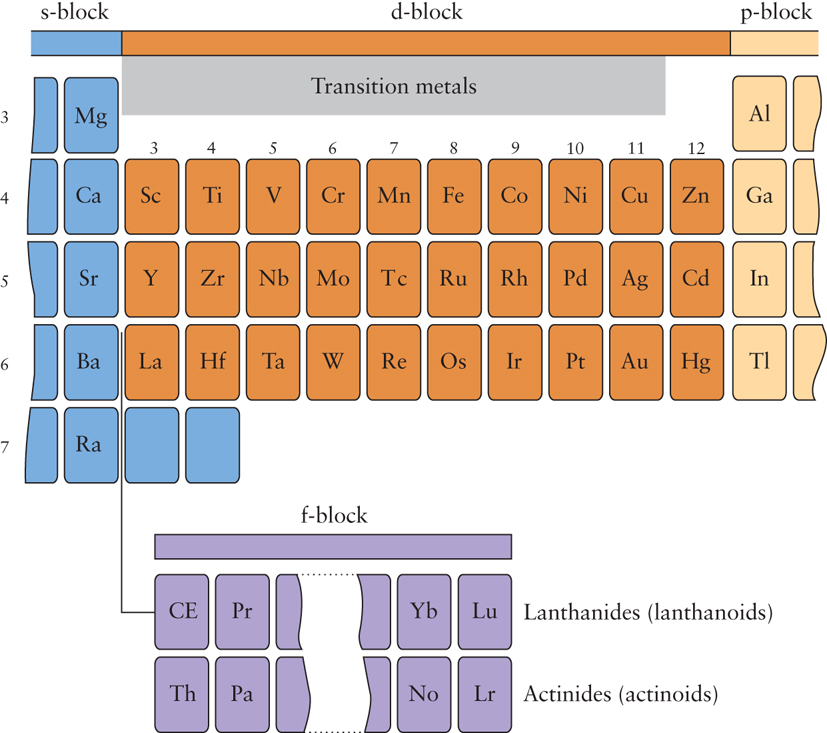
The d block elements are found in the centre of the periodic table. The d -block elements lies in between s- and p-block elements in the long form of the periodic table. Groups 3-12 are called the transition elements. Across any period from Group 3 through 12, the properties of the elements change slightly as appose to drastic change do across a period of representative elements. As you move across the d block, five d orbitals are filled, up to a total of ten electrons. The collective transition metals (or elements) and d block elements are sometimes used in the same context. They don’t – there’s a subtle difference between the two terms. The elements in the Periodic Table which correspond to the d sublevels filling are called d block elements. There are discrepancies between the various syllabuses, but the majority use the definition: A transition metal can form one or more stable ions containing incompletely filled d orbitals.
These metallic elements that serve as a bridge, or transition, between the two sides of the periodic table and are sandwiched between s block and p block. Transition metals have characteristic properties e.g. coloured compounds, variable oxidation states. These are due to the presence of an inner incomplete d sub-shell. Electrons from both inner d sub-shell and outer s sub-shell can be involved in compound formation. Not all d block elements have incomplete d sub-shells. Scandium is a transition metal because it has incompletely filled d orbitals in the ground state while Zinc has a full d10 configuration in ground state as well as in is common oxidation state
- e.g. Zn has electronic configuration of [Ar]3d104s2, the Zn2+ ion ([Ar] 3d10) is not a typical transition metal ion
- Sc forms Sc3+ which has the stable electronic configuration of Ar. Sc3+ has no 3d electrons
Likewise, a transition metal is defined as an element which forms at least one ion with a partially filled d orbital. In period 4 only Ti-Cu are transition metal. Zn, Cd and Hg are not considered as transition elements but they certainly belong to d block. They do not have vacant d-orbitals either in the atomic state or in any stable oxidation state. Copper (Z = 29) can exhibit a +2 oxidation state wherein it will have incompletely filled d-orbitals (3d), hence a transition element. Silver (Z = 47) can exhibit a +2 oxidation state wherein it will have incompletely filled d-orbitals (4d), hence a transition element.
