Origin of Pressure
Pressure is explained by kinetic theory of gases as arising from the force exerted by molecules or atoms impacting on the walls of a container.
In Newtonian mechanics, if pressure is the force divided by the area on which the force is exerted, then what is the origin of pressure in a gas? What forces create pressure? We can understand pressure (and temperature as well) far better from the kinetic theory of gases, which presumes that atoms and molecules are in constant random motion.
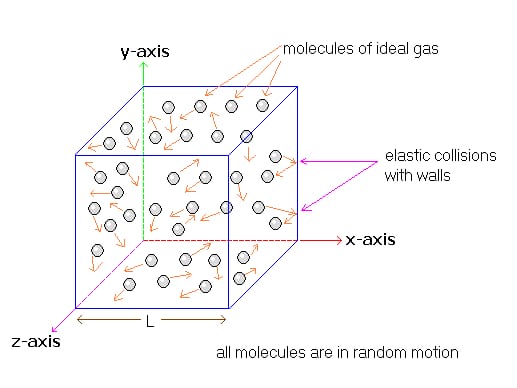
Microscopic Origin of Pressure
Pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as illustrated in the figure below.
Suppose a gas of N molecules, with every mass of m, enclosed in a cubical container of volume V=L3. When a gas molecule collides with the wall of the container perpendicular to the x coordinate axis and bounces off in the opposite direction with the same speed (an elastic collision), then the momentum is lost by the particle and is often gained back by the wall (Δp) is
Translational Motion of Helium: Real gases don’t perpetually behave consistently with the best model underneath certain conditions, such as high pressure. Here, the dimensions of helium atoms relative to their spacing is shown to scale underneath 1950 atmospheres of pressure.
ΔP = Pi, x − Pf, x = Pi, x − (− Pi, x)
= 2Pi, x = 2mvx
Where vx is that the x-component of the initial velocity of the particle.
The particle impacts one specific sidewall once every Δt = 2L / vx,
(Where L is the distance between opposite walls). The force due to this particle is:
F =Δp / Δt = mv2/x / L
The total force on the wall, therefore, is:
F = Nmv 2x / L,
where the bar denotes a median over the N particles.
Since the assumption is that the particles move in random directions, if we divide the velocity vectors of all particles in three mutually perpendicular directions, the average value of the square rate on every direction should be the same. (This doesn’t mean that every particle continuously travels in forty-five degrees to the coordinate axes.)
Pressure: Pressure arises from the force exerted by molecules or atoms impacting on the walls of a container. This force is exerted on an area of L2. Therefore the pressure of the gas is:
P=F / L2=Nmv2 / 3V=nmv2 / 3,
Where V=L3 is the volume of the box. The fraction n=N/V is that the number density of the gas. This is a primary non-trivial result of the kinetic theory of gases because it relates pressure (a macroscopical property) to the common (translational) kinetic energy per molecule which is a microscopic property.
