Usually, the alkenes exhibit a wide range of electrophilic addition reactions. The addition of hydrogen halides such as the hydrogen chloride and hydrogen bromide is a significant example of the electrophilic addition reaction of the alkenes.
Generally, the electrophilic addition to the alkenes starts with the process that pi electrons attack an electrophile and make a carbocation on the most stable carbon. After that, a nucleophile attacks the carbocation and the product is formed. There are various kinds of such additions and they include hydroxylation, hydrogenation, halogenation, oxidative cleavage, hydration, epoxidation, cyclopropanetrione, and the halohydrin formation. Numerous products can be formed as a result of electrophilic addition mechanism.
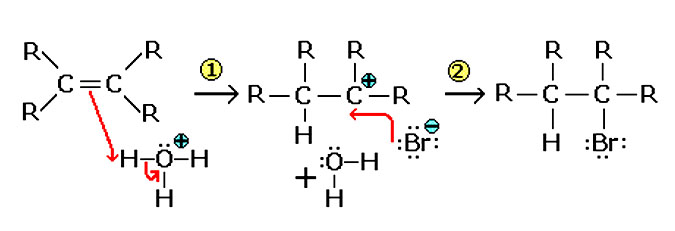
In this process, initially a substrate is attacked by an electrophile and it results in the overall addition of the simple molecules across the multiple bond. The process of electrophilic addition covers the symmetrical alkenes such as cyclohexene and ethene and in the symmetrical alkene same groups are attached to the bond ends of the carbon to carbon double bond. It is an addition reaction in the chemical compounds and is widely studied in organic chemistry. The substrates of the electrophilic addition reaction should have double or triple bonds. Formation of an X+ electropositive ion is the driving force for this addition reaction that makes the covalent bonds with the electron rich unsaturated carbon-carbon double bond. In this reaction a carbocation is formed as the positive charge on the X is transferred to the carbon-carbon double bond.
In the second step of the electrophilic addition, the intermediate which is positively charged combines with the Y that is electron rich and usually, with an anion to make the second covalent bond. The second step is the same as the process of nucleophilic attack that is found in the SN1 reactions. The exact nature of the positively charged intermediate and that of the electrophile are not always clear and are dependent on the reaction conditions and the reactants.In some of the electrophilic addition reactions, such as those between the alkene and hydrogen bromide, there is a choice that to which carbon is bonded to the hydrogen and which one is bonded to the bromine. The electrophilic addition reactions for the unsymmetrical alkenes can be explained based on their structure.
Electrophilic addition gives the Markovnikov Products, where the nucleophile is added to the more highly substituted carbon. This is due to the reason that the intermediates of the carbocation are significantly stabilized by the alkyl substituents. In the process of electrophilic addition, electrophile with the positive charge is affecting the overall formation of the structure, which also bears the positive charge and to make up the conditions for the new addition which in turn leads to the formation of the intermediate bearing that positive charge. To understand the electrophilic addition, this intermediate is the key, and it is significantly important due to the positive nature of the involved particles. If this process is done, then it is possible to understand these reactions as positively charged reactions. Thus, the end product contains the complete structure with the addition of the Y nucleophile.
