d and f Block Elements
Metallic character
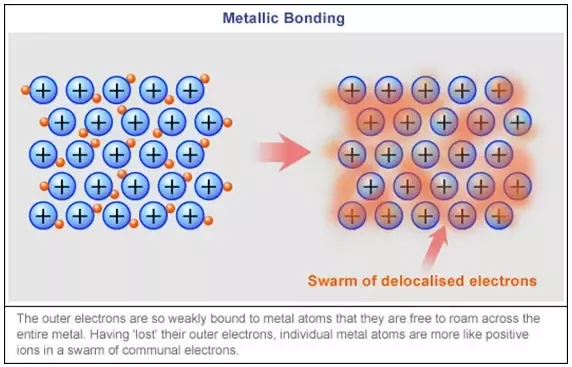
Transition metals are capable to release e- into the pool of mobile electrons from both outer and inner shells. Strong metallic bonds are formed between the mobile pool and the delocalised +ve metal ions.
- High electrical and thermal conductivity
- High melting point
- Malleable – easily beaten into shape
- High tensile strength means that the metal can hold large loads without breaking
- Ductile shows how easily am metal is drawn into wires
These properties are explained by a strong metallic bonding. The 3d and 4s electrons are close to each other in terms of energy level. They become a part of the delocalized sea of electrons that holds the metal lattice together. Alloys are very important, they inhibit slip in crystal lattice usually results in increased hardness and reduced malleability.
Most are good electrical conductors-silver is the best electrical conductor of all elements. Most are malleable, ductile, lustrous, and silver-white in colour, the exceptions: copper is red-brown, gold is yellow, and mercury is a liquid at room temperature. Their melting and boiling points are higher, Melting and boiling points of transition metals are generally high. A large number of unpaired electrons take part in the metallic bonding so these elements form very strong metallic bonds and hence high m.pt & b.pt. the d-orbitals lobes affect the properties in two ways:
1.) electrons in the lobes are far apart and weakly repealing each other.
2.) d-orbitals are poor at shielding because their electron density is low near the nucleus.
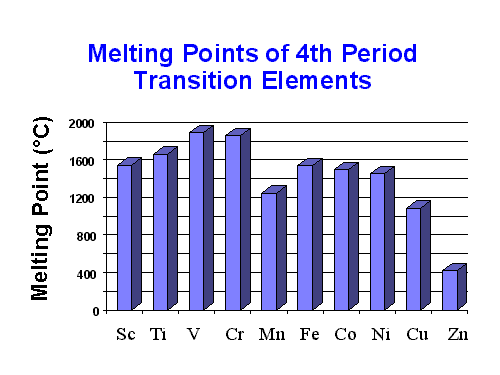
From Sc to V, the m.p and b.p increases due to the increase in a number of unpaired electrons which increases the electrostatic attraction and the metallic bond strength. The opposite happens for Fe to Cu
Cr. has one electron in its s orbital compared to SC-V which have 2 each and that decreases the number of valence electrons thereby reducing metallic strength. Mn, on the other hand, has a half-filled 3d subshell and filled 4s shell which gives the metal extra stability and decreased the number of delocalized electrons. The lower number of delocalized electrons cause the metallic strength to decrease. For the same reason of completely filled 3d shell in Zn, it has lowest m.p and b.p.
Zn, Cd and Hg metals have lower melting and boiling points as they have completely filled d orbitals because of which no unpaired electron is available. Because of the unavailability of unpaired electrons, these metals do not undergo covalent bonding. Rest of the transition metals can pertain metallic as well as covalent bonding depending on the electronic configuration.
Uses of 3d transition metals are listed below:
- Scandium – chemistry strongly resembles lanthanides
- Titanium – excellent structural material (lightweight)
- Vanadium – mostly in alloys with other metals
- Chromium – important industrial material
- Manganese – production of hard steel
- Iron – most abundant heavy metal
- Cobalt – alloys with other metals
- Nickel – plating more active metals; alloys
- Copper – plumbing and electrical applications
- Zinc – galvanizing steel
