Alkynes – method of preparation
The alkynes are prepared by the following general methods:
1. The Action of water on calcium carbide: Ethyne is prepared in the laboratory as well as on industrial scale by the action of water on calcium carbide. For this purpose required calcium carbide is obtained by heating calcium oxide (from limestone) and coke in an electric furnace at 2275K.
In the laboratory, ethyne is commonly prepared from calcium carbide. In this method, a few pieces of calcium carbide are taken in a conical flask fitted with a dropping funnel and a delivery tube. The air present in the flask is replaced by oil gas because acetylene forms an explosive mixture with air. Water is taken in the dropping funnel and allowed to fall into the flask dropwise. The reaction takes place vigorously and ethyne gas is produced which is collected over water.
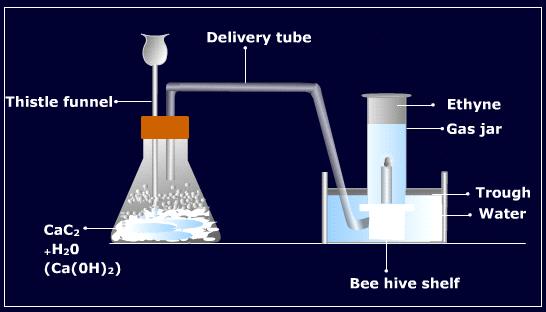
Purification: The gas obtained by the above method generally contains the impurities of hydrogen sulphide and phosphine due to the contamination of calcium sulphide and calcium phosphine due to the contamination of calcium sulphide and calcium phosphide in calcium carbide. These impurities are removed by bubbling the gas through acidified copper sulphate solution.
2. By dehydrohalogenation of dihalides or vicinal dihalides: alkynes are prepared by double dehydrohalogenation of vicinal dihalides means who contains two halogen atoms on the adjacent carbon atoms with the strong base like an alcoholic solution of KOH or sodamide in liquid ammonia. The reaction, in fact, occurs in two steps. The first step gives haloalkane and under suitable conditions, this may be isolated. For example, vicinal dihalides on treatment with alcoholic potassium hydroxide give alkenyl halide.
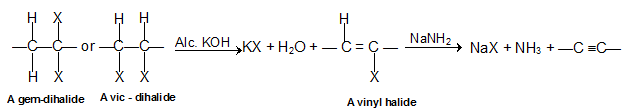
3. By the action of zinc on tetrahalogen derivatives of alkanes (tertrahalides): On treatment with zinc in methanol tetrahalides get dehalogenated to give alkynes. This method is mainly used for the purification of alkynes or the protection of a triple bond because the tetrabromides are themselves obtained from alkynes.
4. Higher alkynes from acetylene: Higher alkynes are prepared from the acetylene by treating its sodium salt with an alkyl halide. The sodium salt is prepared by treating acetylene with sodium metal at 475 K or by the action of sodamide on acetylene in liquid ammonia at 196 K.
5. By electrolysis of the aqueous solution of the potassium salt of fumaric acid: Acetylene is prepared by the electrolysis of an aqueous solution of the potassium salt of maleic acid or fumaric acid.

6. By dehalogenation of haloforms: Chloroform and iodoform on heating with silver powder undergo dehalogenation to form ethyne.
7. Synthesis from carbon and hydrogen: Acetylene can be prepared by passing a stream of hydrogen through an electric arc struck between carbon electrodes at 3270 K.
