The present form of the periodic table
The present song of periodic table is based on the electronic configuration of the element and it’s also called the long form of the periodic table. It contains 18 vertical columns called groups and 7 horizontal rows called periods. Elements in a group have gradation and their properties due to the attraction of the nucleus since the outer valence electron moves further away as we go down the group. Each group signifies identical outer shell electron configuration while each period signifies filling of a new shell. The first period has 2 elements that fill up the K shell. The second period has 8 elements that fill up the L shell. The third period has 8 elements that fill up the M shell. The fourth period has 18 elements that fill up the N shell. The fifth period has 18 elements that fill up the O shell. The sixth period has 32 elements and in this period, the electrons start filling 4f-orbital after 5d-orbital accommodate one electron. The 7th period is incomplete.
The most significant feature of the long-form periodic table is that it facilitates in understanding the cause of periodicity of properties and why at regular intervals similar properties reoccur in elements. In this periodic table, the position of hydrogen still remains uncertain and the inner transition metals such as lanthanides and actinoids are placed separately from the main body of the table. (Singh, 2011).
The present periodic table has a foul division of s, p, d and f block and each of these blocks corresponds to the outermost electronic configuration of elements that are placed within those blocks in the periodic table (Huheey, Keiter, Keiter, & Medhi, 2006).
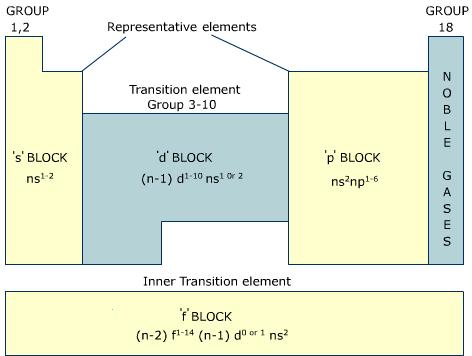
Figure 1: Location of four blocks of elements s, p, d, f in the long form periodic table.
The s-block metals are soft and they have a low melting and boiling point. These metals are very reactive and highly electropositive. These metals lose their valence electrons to form covalent or divalent cation making them strong reducing agents.
The p-block elements make chemical compounds by losing gaining or sharing valence electrons. The halogens are highly reactive with s-block elements and most of the other p-block elements form covalent compounds. However, elements in group 18 are inert and they show no form of reactivity.
The d-block elements have partially filled d orbitals and they are called transition elements. Consequently, elements in this block tend you have variable oxidation states. For example, Fe2+ and Fe3+; Cu+ and Cu2+. Transition elements can be either highly reactive electropositive alkali or covalent compounds. They also have high melting and boiling point. Most of the d-block elements form colored salts and their compounds are generally paramagnetic.
The f-block metals also have high melting and boiling point and they also exhibit variable oxidation state. Most of these elements absorb light from certain visible wavelength making their salts colored. Actinides are highly radioactive elements.
Table 1: Blocks in the periodic table
| Block | Group | Electronic configuration |
| s-block | Group 1 (Alkali metals) Group 2 (Alkaline earth metals) | ns1 ns2 |
| p-block | Group 13-18 (Non-metals and Nobel gases) | ns2 np1-6 |
| d-block | Group 3-12 ( Transition metals) | (n-1)d1-10 ns1-2 |
| f-block | Lanthanoids and Actinoids | (n-2) f1-14 (n-1)d0-1 ns2 |
