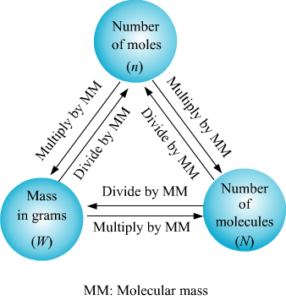
Substances around us take up some space and have mass. These substances are made up of molecules. Molecules need to be measured in various experiments and here it is necessary that the measurements are accurate. Using calculations scientists determine the number of moles a particular reaction will require. Once the number of moles required are known, we can use the concept of molar mass to calculate how many grams of substance is required. Molar mass also called the molecular mass is defined as the sum total of mass in grams of all the atoms that make up a mole of a particular molecule. It is therefore the relative mass of a molecule expressed in atomic mass units (u).The unit to measure it is grams per mole.
| Common symbols | M |
| SI unit | kg/mol |
| Other units | g/mol |
How to find molar mass of a compound:
Compounds are nothing but substances that are made up of more than one element. The compound sodium chloride is made up of two elements, namely, sodium and chlorine. Here we will use this compound, sodium chloride, as our first example on how to calculate the molar mass for the compounds. What we need to do first is, we need to get aperiodic table and find sodium and chlorine in that periodic table.
Step 1: Find the atomic masses of individual elements in the periodic table.
The very first thing is we need to find the individual atomic masses for each element. If you look at the entries for sodium and chlorine in the table
Sodium has an atomic mass of 22.98976 g/mol.
Chlorine has an atomic mass of 35.453 g/mol.
Step 2: Count how many atoms there are for each element
For the compound sodium chloride, since there are no subscripts (small numbers at the bottom of each element’s symbol), that means there is only one sodium and only one chlorine atom for this compound.
Step 3: Find the molar mass
Now that we know how many atoms there are for each element, we can find the molar mass.
First, we will calculate the mass of the sodium atom, which is 22.98976 grams per mole. Next, we will do the same for the mass of chlorine atom, which is 35.453 grams per mole. We then add these two masses together to find the total mass of sodium chloride molecules. This will come out to be 58.44276 grams per mole.
Let’s see a solved example to understand the concept:
Question: Find out the relative molecular mass of water (H2O). (b) Calculate the molecular mass of HNO3.
Solution:
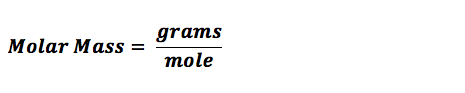
(a) Atomic mass of hydrogen = 1u, oxygen = 16 u so the molecular mass of water, which contains two atoms of hydrogen and one atom of oxygen is = 2 × 1+ 1×16 = 18 u
(b) The molecular mass of HNO3 = the atomic mass of H + the atomic mass of N+ 3 × the atomic mass of O = 1 + 14 + 48 = 63 u
