Introduction to ethers
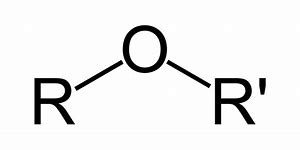
Ethers are compounds containing the ether group in them. The ether group is R-O-R’ type of the compound. In which the R and R’ are alkyl groups. The alkyl group might be similar or not in accordance top the type of ether.
Due to the presence of alkyl groups the electron density on the oxygen atom increases subsequently. This is because the alkyl groups are the electron donating type of groups. Thus, subsequently the ethers are themselves also the electron donating groups.
Nomenclature of ethers:
The ether group of IUPAC nomenclature does not have a proper suffix name. The suffixes are decided by the alkyl groups attached to the oxygen atom. The –OR group is termed as alkoxy group. Where the R group could be any alkyl like methyl, propyl , butyl ,etc.
In the nomenclature of ethers of R-O-R’ type, the smaller alkyl group, becomes the Alkoxy group according to the nomenclature. For example, in the compound CH3-CH2-O-CH3, the smaller alkyl group is the CH3 group that is methyl group. Hence, the alkoxy group would be methoxy by name. Now, for naming the Ether completely we have to address the entire compound. So, now after naming the Alkoxy group names the other alkyl group as it is. Therefore, for the compound CH3-CH2-O-CH3, the IUPAC nomenclature name would be Methoxy ethane.
Even in the cis or trans type of geometry of compounds the ground rules of naming the ethers remains the same.
For ethers with same type of alkyl groups on both the sides of oxygen the nomenclature is also different. In type of ethers we generally make the use of the word ‘di’ in the nomenclature. After using the word ‘di’ we subsequently write the name of the alkyl group and at the end we write ether.
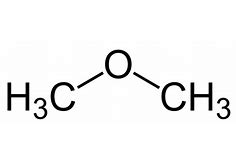
For example, for the ether CH3OCH3, there are two methyl groups attached on both the sides of oxygen respectively. Hence the nomenclature of the ether will be ‘dimethyl ether’.
Since the ethers could be attached at any position in the compound, it has to be named along with the number of carbon to which it is attached. The numbering of the carbon atoms is to be done in accordance to the preference in IUPAC nomenclature.
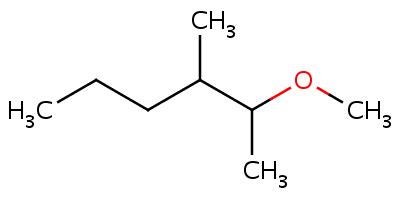
In this compound, as the numbering of carbons is done according to the preference of the carbon atoms, the carbon with ether gets number as 2. The lower alkyl group attached to the ether group is methyl. Therefore, the alkoxy name of the ether will be methoxy. Hence, the IUPAC nomenclature of the compound will be 2-methoxy 3- methyl hexane.
