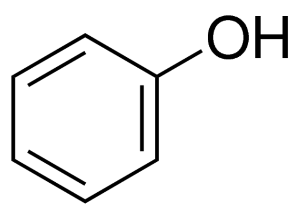
Phenols are organic hydrocarbon compounds with a hydroxyl group directly attached to an aromatic hydrocarbon compound like Benzene Ring. Phenols are also termed as Phenolic compounds. The molecular formula of phenols is C6H5O6. The synthesis of Phenols is Natural as well as artificial.
Nomenclature
Phenol is the common name of the C6H5O6 compound. By definition, the phenol compound will be known as Hydroxybenzene. It is named in the same manner as the IUPAC naming method for aliphatic alcohols.
In accordance with the IUPAC nomenclature of the compound, the parent name of the compound is termed as Benzol. Along with the hydroxy group, several different molecules can be subsequently attached to different positions of Ortho, Meta or Para of the phenol compound. Whereas the common name of the compound always remains as Phenol. In preference to different molecules attached to the compound, the numbering is always started with the OH group of the phenol. After that, according to the highest preferences of different molecules the numbering is done.
Examples
In some nomenclature of Phenols, the compound is also termed with their common names like the Cresol compound. In this compound the CH3 compound is attached at the ortho, meta or para positions and are named as o-cresol, m-cresol and p-cresol respectively.
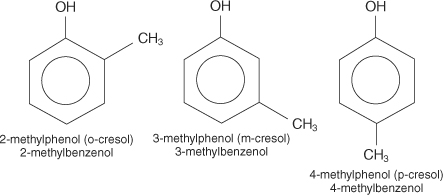
In accordance with the nomenclature with the IUPAC system, certain ground rules are to be followed respectively. For naming any Phenol compound the numbering of carbon atoms has to start with the OH group. Hence starting with the OH the group the CH3 compound will get 3, 4, and 5 positions in o-cresol, m-cresol, and p-cresol respectively.
For the o-cresol compound, the OH group gets 1 and the methyl group is attached at 2nd carbon, therefore the IUPAC name of the compound will be 2-methylphenol. Similarly, in the m-cresol compound the CH3 group is attached at the 3rd position in the compound, therefore the IUPAC name of the compound will be 3-methylphenol or 3-methylbenzol. Similarly, in the p-cresol the methyl group been attached at the 4th position leads to the nomenclature name of 4-methylphenol.
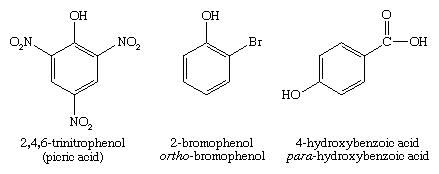
In this example as well, we may follow the rules for the Nomenclature of phenols. In the first example, we have the Picric acid, in which 3 nitro groups are attached at both ortho positions and the para position respectively. So, by starting to number from the OH group, the NO2 group might get the 2,4 and 6 positions respectively. Therefore, the name of the compound will be 2,4,6 trinitrophenol.
Similarly, for the 2nd compound the Br (Bromine) group is attached in the 2nd position. Therefore, the IUPAC name of the compound will be 2-bromophenol. Also, for the 3rd compound the IUPAC name will be 4-hydroxybenzoic acid. This difference in the nomenclature will be because the COOH group has more preference than the OH group.
