Introduction to Number of Atoms in a Unit Cell
According to our knowledge, there are several unit cells in a crystal lattice. In the unit cell, each of the constituent particle comprising of a molecule, ion, or atom carries a fixed and specific position, known as the lattice site.
We can easily do the calculation of the number of molecules/atoms and ions in the unit cell by examining and determining constituent particles’ position and nature in the unit cells.
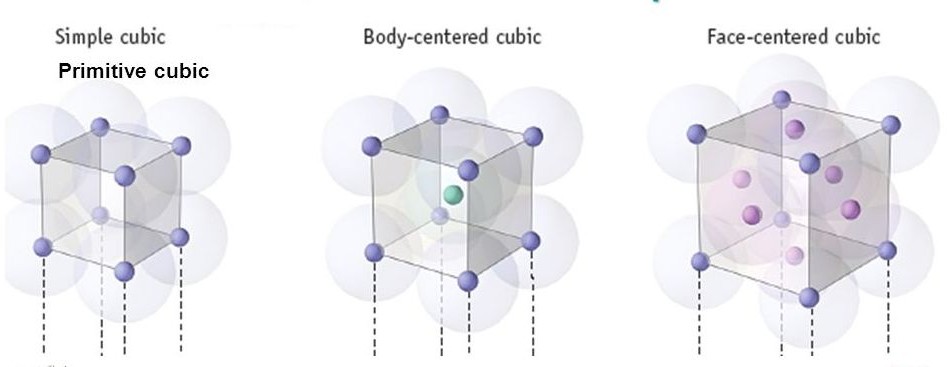
Fig 1: Types of Unit Cell
Primitive Cubic Unit Cell
In Primitive Cubic Unit Cell, the atoms are located only at the corners. This means that 8 atoms are placed on the lattice at 8 corners. Located at the corner, each atom contributes about 1/8 of the original cell’s volume. Therefore, since there’s a net sum of eight (8) atoms present in the primitive cubic unit cell, the primitive cubic unit cell’s total number of atoms is given by:
![]()
Thus, there’s 1 atom only present in the primitive cubic unit cell.
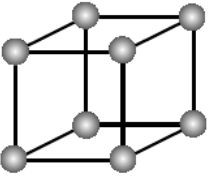
Fig 2: The corners acquires 1/8th part only of the atom
Body- Centred Cubic Unit Cell
In a Body-Centred Unit Cell, at 8 corners, 8 atoms are placed and 1 atom is located at structure’s centre. Thus, Body-Centred Unit Cell’s total atoms is given by:
Seeing that, on the corners, 8 atoms are present and each atom contributes 1/8 of the original cell’s volume. Accordingly, the total atoms of Body-Centred Unit Cell will be:
There are two corners present; one having 8 corners and another is sharing 1/8th volume of the whole cell, so
![]()
In addition, the atom located at the centre is entirely present at the cell’s centre and which cannot be shared.
1 × 1 = 1 atom
Thus, in total, there’re two atoms in the Body-Centred Unit Cell.
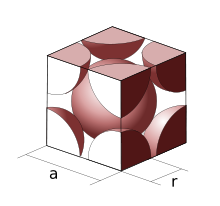
Fig 3: Atoms in Body-Centred Unit Cell
Face-Centred Cubic Unit Cell
The face-centred cubic unit cell is having atoms that are present at the centre of all faces and at 8 corners. Additionally, each atom placed on unit cell’s centre is being shared by 2 adjoining unit cells. Thus, half of the atoms only refer to a Single Unit Cell.
Therefore, in Face-Centred Cubic Unit Cell
At 8 corners, 8 atoms are placed, thus, each corner will gain 1/8 part of an atom
![]()
With six faces present, each face gains 1/2 part of the atom, then
![]()
The net sum of atoms in a Face-Centred Unit Cell = 1 + 3 = 4 atoms
End-Centred Cubic Unit Cell
In the End-Centred Cubic Unit Cell, in a cube, on the 8 corners, 8 atoms are present. One atom each is having its presence on cube’s 2 opposite faces.
Thus, in the End-Centred Cubic Unit Cell,
On 8 corners, 8 atoms present, as a result, each of the atoms contributes a 1/8th cells’ part.
![]()
At the cell’s centre, two atoms are present. Each atom is making a contribution of 1/2th cell’s portion/part.
![]()
The total of atoms located in the End-Centred Cubic Unit Cell = 1 + 1 = 2 atoms
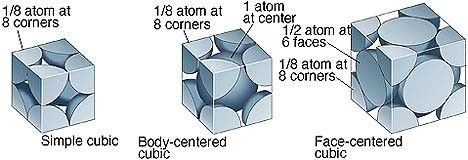
Fig 4: The Contribution of Atom in various unit cells
Given below is the table representing the total number of atoms in a unit cell, in a summarized form
| Type of Unit Cell | Number of atoms at corners | Number of atoms on faces | Number of atoms in center | Total |
| Simple Cubic | 8 × 1/8 = 1 | 0 | 0 | 1 |
| Body Centred Cubic | 8 × 1/8 = 1 | 0 | 1 | 2 |
| Face Centred Cubic | 8 × 1/8 = 1 | 6 × 1/2 = 3 | 0 | 4 |
| End Centred Cubic | 8 × 1/8 = 1 | 2 × 1/2 = 1 | 0 | 2 |
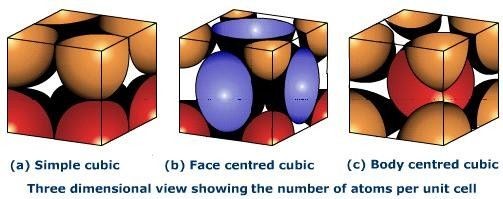
Fig 5: Types of Unit Cell
