p-Block Elements: Group 18
Occurrence
All the noble gases except radon occur as monoatomic gases in the atmosphere. These are the only gases in earth’s atmosphere that are monoatomic since most other gases are diatomic. Together they make up 1wt% of the atmospheric air. Argon is the third most abundant gas in the atmosphere after N and O. All of the group 18 elements except He and Rn can be extracted by the fractional distillation of liquid air. The major source of helium is from the cryogenic separation of natural gas.
After Hydrogen H, Helium (He) is the second most abundant element in the universe although it is found rarely on earth because of its lightweight. It is so light that it can reach the high speeds required for escaping from the atmosphere. Helium is found with natural gases that are trapped under rock formations. It gets collected as a result of the emission of α particles by radioactive elements. He is the only element known to have more than one liquid phase. Helium is the only substance known to man which does not solidify when cooled at normal pressure.
Argon found in nature makes up to 0.947% of dry air. It is the most abundant noble gas found in dry air and its concentration increases above sea level. The solubility of argon in water is more than nitrogen which makes it available in sea and river water. This is the reason behind the presence of argon gas in the gas-bladder of fishes. It is found in the free state in plants and blood cells of animals.
Neon is widely distributed in the universe and it is the fifth most abundant element in the universe. Neon can be created at high temperatures by the thermonuclear reaction between oxygen and carbon which occurs in star cores. Many stars have stable isotopes of neon due to this reaction.
Only one known stable and neutral compound of Krypton is known to date. Krypton fluoride, KrF2 obtains the oxidation state of +2. a
Xenon is present is a traces in the atmosphere (less than one ppm by volume) and it is found in some mineral springs. Commercially it is obtained as a by-product of separation of air into oxygen and nitrogen. Xe can form 8 naturally occurring stable isotopes and unstable isotopes >40 that undergo radioactive decay. The atmosphere of planet Jupiter contains xenon in high quantity. Metallic xenon is produced by applying several hundred kilo bars of pressure. Most of the known xenon compounds contain strongly reducing fluorine or oxygen atoms where it can obtain +2 to +8 oxidation state. Xe shows a tendency to lose electrons in many of its reactions. Therefore, Xe combines with only more electronegative elements like F and O or electronegative groups. Xe does not combine with less electronegative elements like Cl2 or N2.
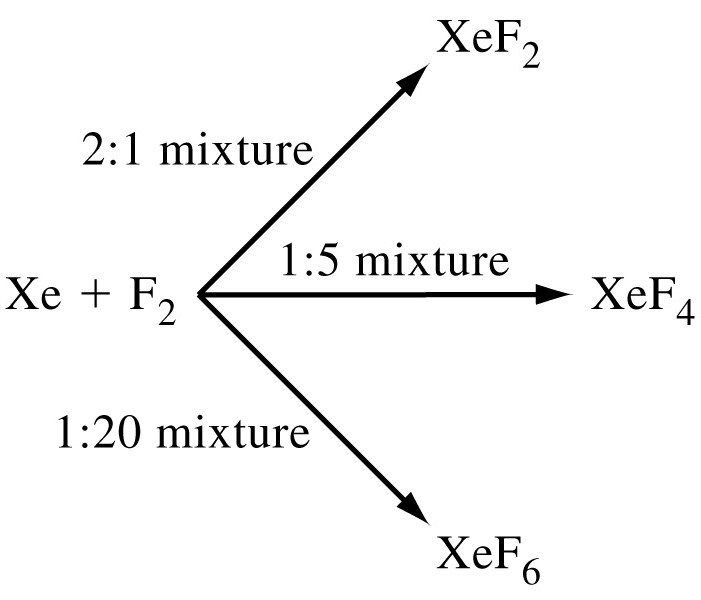
Radon, a radioactive noble gas, is produced from the radioactive decay of heavier elements, including radium, thorium, and uranium. Element 118 (Og) is a man-made radioactive element, produced by striking a target with accelerated particles.
Radon is also present in very small amounts in soils and minerals and it can cause health hazards when inhaled.