The oxidation number of an atom is a number that represents the total number of electrons lost or gained by it. It describes the degree of oxidation of an atom in a chemical compound. It has been developed to keep track of electron shifts in chemical reactions involving formation of covalent compounds. In this method the assumption made is that there is a complete transfer of electron from a less electronegative atom to a more electronegative atom.
Oxidation state of an element in a compound is denoted by Oxidation number according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to more electronegative element.
A set of rules has been formulated to determine the oxidation number of an element in a compound or ion. This is because it is not always possible to remember or make out easily in a compound/ion, which element is more electronegative than the other. The rules are as follows:
- In case of elements in the Free State, each atom bears an oxidation number of zero. Evidently each atom in H2, O2, Mg, Al has the oxidation number zero.
- For ions which are composed of only one atom, the oxidation number is equal to the charge on the ion. Thus Na+ ion has an oxidation number of +1, Mg2+ ion, +2, ; and so on. Aluminium is regarded to have an oxidation number of +3 in all its compounds.
- In most of its compounds oxygen has an oxidation of -2. But, in the case of peroxides, the oxidation number corresponding to oxygen is -1
- Except when it is bonded to metals in binary compounds, the oxidation number of hydrogen is +1. For example in NaH, and CaH2, its oxidation number is –1.
- All group 1 elements(alkali metals) have an oxidation state of +1 in their compounds.
- All group 2 elements (alkaline earth metals) exhibit an oxidation state of +2 in their compounds.
- A halogen (group 17 elements) has an oxidation number of -1 assigned to them in the compounds made up of two elements.
- The sum of the oxidation number of all the atoms in a compound must be zero. In case of polyatomic ion, the sum of all the oxidation numbers of atoms of the ion must equal the charge on the ion.
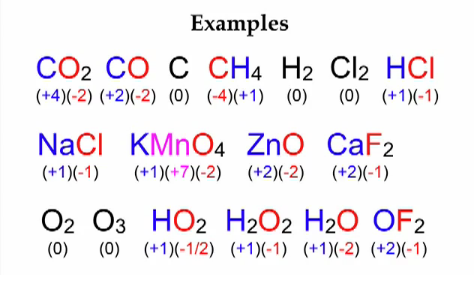
Through the application of above rules, we can find out the oxidation number of the desired element in a molecule or in an ion. It is clear that oxidation number of metallic elements is positive and that of non-metallic elements is positive or negative. Usually positive oxidation states are displayed by atoms of transition elements. The highest oxidation number of an illustrative element is the group number for the first two groups and the group number minus 10 for the other groups. Thus, it shows that the highest value of oxidation number manifested by an atom of an element generally increases across the period in the periodic table.
