p-Block Elements: Group 15
Phosphorous: allotropic forms, compounds of phosphorus
Phosphorous is used extensively in pyrotechnics, steel making and alloy making while phosphoric acid makes an essential part of fertilizers. Phosphorous is also used in electrode-less plating.
Phosphorous is naturally found in three allotropes:
- White Phosphorus = P4 (tetrahedral) – very reactive
- Black Phosphorus = crystalline structure – much less reactive
- Red Phosphorus = amorphous with P4 chains
White P is stored underwater to protect from the air because it reacts vigorously with oxygen so it must be stored under water. Red and black P for a stable chain like structure in air, burn on heating. P is soluble in organic solvents.
White phosphorus reacts with sodium hydroxide and water to produce its salt, sodium hypophosphite.
P4 +3NaOH + 3H2O PH3 + 3NaH2PO2
Red phosphorus is produced by heating white phosphorus at 573K in an inert atmosphere. This process may take several days to let the transformation to occur and create a crystalline structure with more metallic bonding. It has a chain-like structure. Black phosphorous has two forms alpha-black phosphorous and beta-black phosphorous. Alpha black is formed when red phosphorus is heated in a sealed tube at 803K. It can be sublimed in air and has an opaque monoclinic or rhombohedral crystal structure. It does not oxidise in air. Beta black us prepared by heating white phosphorus at 473 K under high pressure. It does not burn in the air upto 673K.
| White phosphorus | Red phosphorus | Black phosphorus |
| Discrete tetrahedral P4 molecules | Polymeric structure consisting of chains of P4 units linked together | Exists in two forms – α black P and β black P |
| Very reactive | Less reactive than white P | Stable compound |
| Has chemiluminescence, Glows in the dark | No chemiluminescence | No chemiluminescence |
| Translucent or white waxy solid | Has an iron-grey lustre | Has an opaque monoclinic or rhombohedral crystals |
| Soluble in CS2 but insoluble in water | Insoluble in water as well as CS2 | Insoluble in water as well as CS2 |
| It has low ignition temperature, therefore, kept under water | High ignition temperature | Very high ignition temperature |
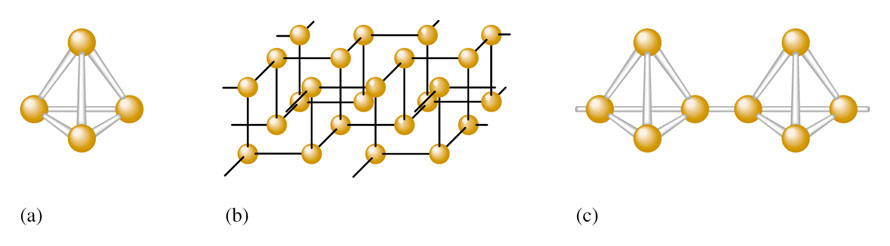 Fig1: (a) P4 molecule found in white phosphorous (b) crystalline network structure of black Phosphorous (c) chain structure of | ||
Phosphorous hexoxide (P4O6)
It has P in its +5 oxidation state. This compound is a powerful drying agent.
Phosphorus pentoxide (P4O10)
It is produced when white phosphorus reacts with oxygen.
P4 + 5O2 P4O10
It is one of the most useful drying agents for industrial purposes. Reacts with water to form phosphoric acid. It has P in its +3 oxidation state.
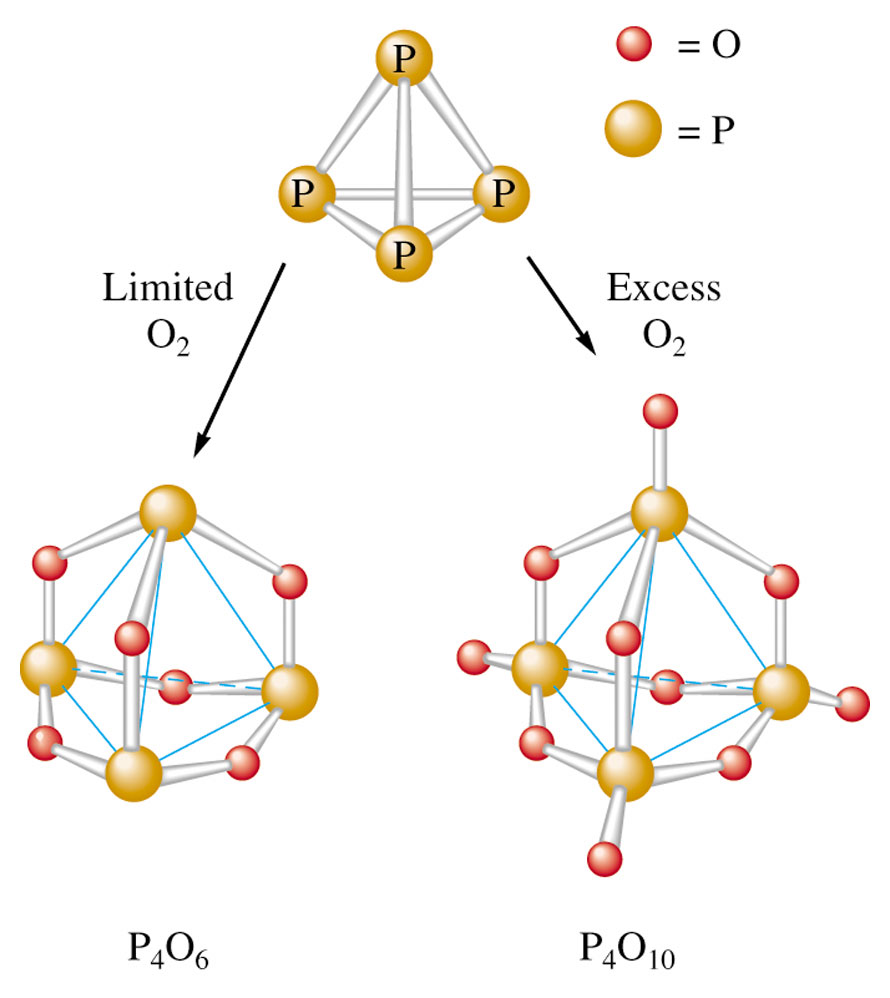
Fig 2: Structures of oxides of phosphorous
The phosphate ion, (PO4)3-, is a tetrahedron ion. It is the conjugate base of phosphoric acid and it is a weak oxidizing agent. This ion forms an essential component of nucleotides. It also forms a bright yellow precipitate with ammonium molybdate:
(PO4)3-+3NH4++12MoO42−+24H+(NH4)3PO4⋅12MoO3+12H2O
Fig 3: The phosphate ion
