Physical Properties
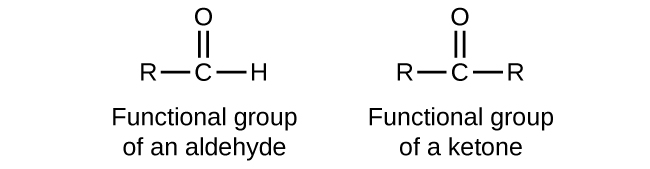
In many of the reactions, ketones and aldehydes are the same as both of them have the carbonyl functional group. In the most important types of reactions such as oxidation, they differ greatly. Ketones resist the oxidation, whereas aldehydes are readily oxidized to the carboxylic acids. Aldehydes are among the organic compounds which are easily oxidized. The ease of oxidation helps in their identification.
Boiling Points
Ketones and aldehydes have higher boiling points than the other compounds which are non-polar. But the boiling points are lower than the corresponding carboxylic acids and alcohols as ketones and aldehydes do not make the hydrogen bonding with themselves.
Solubility
The lower members of the ketones and aldehydes which are having up to four carbons are soluble in the water due to the presence of the hydrogen bonding. However, their higher members are not dissolved in the water, because the part of hydrocarbons is large and it resists the formation of H-bonds with the molecules of water. Also, there are dipole-dipole interactions and the dispersive forces between the water molecules and the aldehydes and ketones.
Chemical Properties
A carbonyl group is present in both ketones and aldehydes, so usually, they undergo the same reactions such as reduction, halogenation, oxidation, and the nucleophilic addition reactions.
Reduction of Aldehydes and Ketones
They can be reduced to a variety of compounds such as hydrocarbons, and alcohols.
Oxidation Reactions
Aldehydes have hydrogen atom on the carbonyl group that can be easily converted to the hydroxyl group, so the aldehydes are easily oxidized to the carboxylic acids. Whereas, in the ketones, no hydrogen atom is attached to the carbonyl group so they cannot be easily oxidized and strong oxidizing agents are required for their oxidation.
Reactivity of the Aromatic Aldehydes and Ketones
Electron donating resonance is exhibited by the aromatic aldehydes and ketones, so electron density is increased on their carbonyl carbon. It causes the carbonyl carbon to be less electrophilic and is less susceptible to the nucleophilic attack. However, the aromatic aldehydes are more reactive than the aromatic ketones.
Bonding and Reactivity
As compared to the carbon, oxygen is far more electronegative and it has a strong tendency for pulling the electrons towards itself in the carbon-oxygen bond. The double bond between carbon and oxygen is highly polar in the aldehydes and ketones. In the carbonyl group, the carbon atom is slightly positive and it can be easily attacked by the nucleophiles.
