Physical Properties of Primary Alcohols
- The number of carbon atoms in primary alcohols is ranging from one to four and as compare to the equivalent alkanes their boiling points are low. Ethanol has the molecular weight of 46 and its boiling point is 78 degrees Celsius, however, the boiling point of propane is -42 degree Celsius and here such a huge difference indicates that the molecules of ethanol are connected much strongly.
- However, it is the fact that the boiling point of alcohol is always higher than the boiling point of the analogous alkane and the boiling points are increased with an increase in the number of the carbon atoms. The patterns in the boiling points strongly reflect the patterns of the intermolecular forces.
- Primary alcohols are completely soluble in the water and if two of them are mixed in any proportion, it produces a single solution. However, with an increase in the length of hydrocarbon chain, the solubility is decreased.
- Primary alcohols are more flammable as the flammability of the alcohols is decreased with an increase in the size and the mass of the molecule.
- At room temperature, most of the commonly used alcohols are colorless liquids. Primary alcohols are non-viscous as the viscosity increases with an increase in the size of the molecule. This is because the strength of intermolecular forces is increased and the molecules hold more firmly at their places.
Chemical Properties of Primary Alcohols
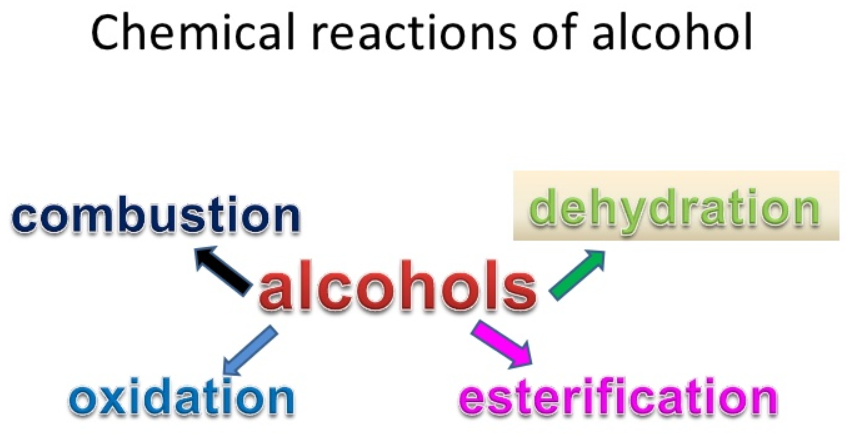
- Primary alcohols undergo the various spontaneous chemical reactions due to the cleavage of O-H and C-O bonds.
- Generally, the alcohols are more acidic than the alkanes and amines but less than the hydrogen halides.
- Ethanol, methanol, and propanol are miscible in the water.
- Alcohols have two regions, the hydrophobic, and the hydrophilic.
- In the presence of oxygen, alcohol burns to produce water and carbon dioxide. They burn easily and cleanly without producing the soot.
- Primary alcohols can be easily oxidized to ketones or the aldehydes and the oxidizing agents are used for this oxidation, such as acidified potassium manganate or acidified potassium dichromate. The further oxidation of the ketones and aldehydes gives the carboxylic acids.
- Their reaction with carboxylic acids produces the esters and they can also be dehydrated to the alkenes by heating them with the concentrated sulphuric acids. In this reaction, water is produced as a byproduct. Usually, alcohols undergo the dehydration if they are treated with the protic acids.
