p-Block Elements: Group 17
Preparation properties and uses of chlorine and hydrochloric acid
Chlorine exists as chlorides in seawater, salt lakes and brine deposits. Chlorine gas is prepared industrially by the electrolysis of sodium chloride solutions. It is also a by-product of the preparation of metals by electrolysis of molten salts such as NaCl, MgCl2 and CaCl2. Elemental chlorine is an oxidizer. It undergoes halogen substitution reactions with lower halide salts. For example, chlorine gas can be bubbled through a solution of bromide or iodide anions. The gas oxidizes these anions to bromine and iodine respectively. Chlorine also participates in free-radical substitution reactions with hydrogen-containing organic compounds.
Chlorine is used to purify water supplies because it is toxic to bacteria, some of which can cause disease. Adding it to water supplies is therefore beneficial for the population. Chlorine is used to purify water supplies because it is toxic to bacteria, some of which can cause disease. Adding it to water supplies is therefore beneficial for the population. Chlorination of drinking water raises questions about individual freedom because it makes it difficult for individuals to opt-out. Chlorine is also used in disinfectants, construction of PVC, chloroform, and as an oxidizing agent in many pharmaceutical products.
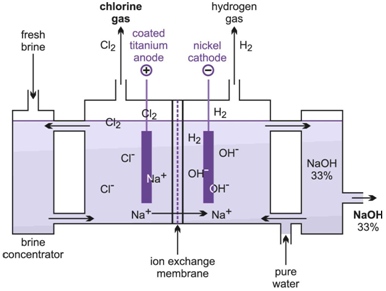
Chlorine can be manufactured at an industrial scale by electrolysis of brine (sodium chloride, NaCl) solution. This process talks place in a diaphragm cell where two co-products are also obtained: caustic soda ( sodium hydroxide, NaOH) and hydrogen gas ( H2).
Cathode : 2 H+ (aq) + 2 e − → H2 (g)
Anode : 2 Cl− (aq) → Cl2 (g) + 2 e−
Overall process : 2 NaCl ( or KCl ) + 2 H2O → Cl2 + H2 + 2 NaOH ( or KOH )
Hydrochloric acid is a colourless, odourless and highly corrosive solution of hydrogen chloride and water. HCl can dissociate once and give up one proton. One of the strongest commercially available cleaners today is HCl. It is extremely powerful and used in masonry, manufacturing of glue and bleaching agent in the textile industry. It is also used in purifying common salt.
In the laboratory, HCl can be prepared by heating sodium chloride (NaCl) with concentrated H2SO4.The gas (HCl) that is produced is dried by passing it into conc. H2SO4 and then mixed with water to produce the acid.
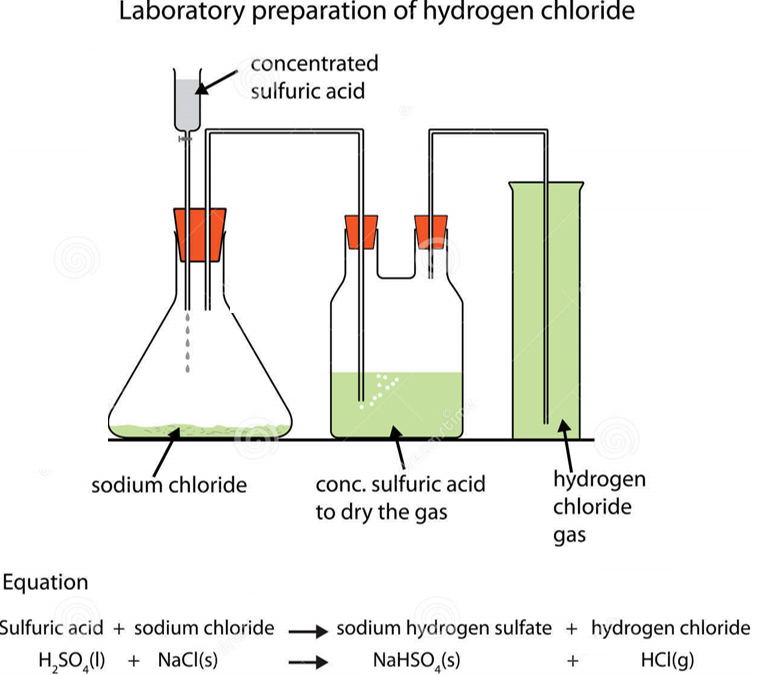
At the industrial scale, it is prepared by
H2O and HCl gas combine to form hydronium cations, H3O+ and chloride anions, Cl– through a reversible chemical reaction:
HCl + H2O → H3O+ + Cl–
The resulting solution is hydrochloric acid and must be handled carefully because it is a strong acid. Hydrochloric acid has a choking, irritating smell. It is very soluble in water, and it turns blue litmus red. Hydrogen chloride gas evolves when a concentrated solution of HCl is heated. At higher temperatures, the solubility of the gas decreases, and vice versa.
